The crystal structure determinations (resolution 2.1 Å) of a series of Ca++, Mn++ containing protein - pea lectin in complex with gluco- and mannopyronoside derivatives have revealed the stereochemical features of the binding site responsible for carbohydrate recognition and binding. Based on the determined structure, residue mutations were proposed to change carbohydrate specificity.
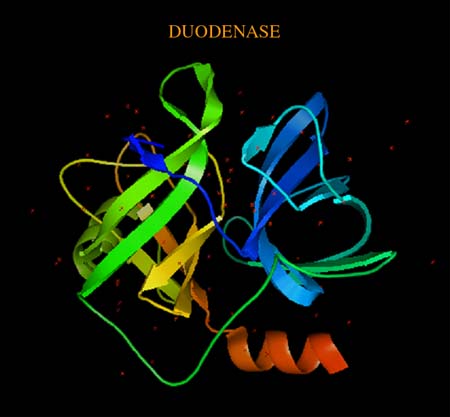
The three-dimensional structure (resolution 2.4 Å) of serine protease and bovine duodenase demonstrated the structural features of the active site compatible with effective accommodation of P1 residues typical of trypsin (Arg/Lys) and chymotrypsin (Tyr/Phe) substrates. These specificities in the past were considered to be mutually exclusive. The computer modeling of the complexes with the corresponding octapeptide substrates confirmed the experimental conclusions. The obtained results may permit design of enzymes with a specific ratio of trypsin and chymotrypsin activities.
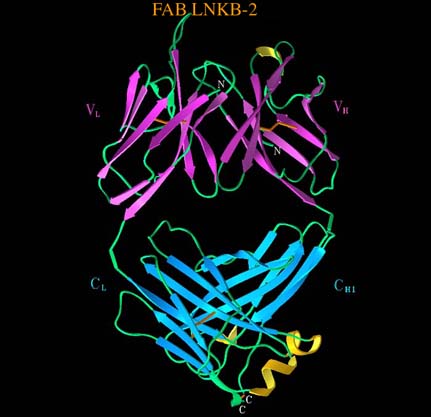
The crystal structure of the Arg32His mutant of the human tumor necrosis factor (TNF-a), an important immune mediator, has been established at 2.5 Å. Models of the structure complexed with P55 and P75 receptors explained the decrease in its cytotoxic activity. The three dimensional structure of the antigen binding fragment of a monoclonal antibody to human interleukin-2 complexed with antigenic nonapeptide has been determined at 3 Å resolution. Antibody-antigen complexation involves a significant rearrangement of the epitope containing region of the interleukin-2 with retention of the a-helical character of the epitope fragment.


Contact: V.Z. Pletnev (pletnev@ibch.ru)