Лаборатория биомедицинских материалов
Группа разрабатывает и изучает различные биодеградируемые материалы (скаффолды) на основе природных и синтетических полимеров для тканевой инженерии и регенеративной медицины При этом такие биоматериалы представлены в различных формах, в частности в виде пленок, нано- и микроволокон, гидрогелей, микрочастиц (микроносителей для роста клеток) Для изучения цитотоксичности и других важных свойств полученных матриксов в модели in vitro используются различные культуры клеток (фибробласты, остеобласты, стволовые мезенхимальные клетки и др). Кроме того, в группе разрабатываются как различные системы доставки противоопухолевых лекарств (наночастицы, мицеллы, липосомы, полиэлектролитные наноконтейнеры и др), так и новые 3D in vitro модели на основе мультиклеточных опухолевых сфероидов для тестирования этих систем. Такие 3D in vitro модели перспективны для исследования механизмов действия противораковой терапии (химиотерапия, фотодинамическая терапия и др) и скрининга новых лекарств, а также средств их доставки непосредственно перед клиническими испытаниями. Их использование позволяет удешевить клинические испытания и минимизировать количество животных, необходимых для этих тестов.
Группа сотрудничает с различными лабораториями ИБХ, а также с Национальным политехническим институтом Лотарингии (Нанси, Франция), Центром биоматериалов Льежского университета (Льеж, Бельгия), Страсбургским университетом (Страсбург, Франция), Институтом Oniris (Нант, Франция), Королевским университетом (Кингстон, Канада) и др.
Группа основана как независимое подразделение в 2017 г., выделившись из Лаборатории полимеров для биологии.
Группа занимается разработкой биодеградируемых матриксов (микроносители, волокна, гидрогели, пленки) для регенеративной медицины (рис. 1.), систем доставки противоопухолевых лекарств (рис. 2), различных 3D-моделей in vitro на основе мультиклеточных опухолевых сфероидов (опухолевые сфероиды в микрокапсулах (рис. 3), на основе сфероидов, полученных из монослойной культуры клеток с помощью RGD- пептидов (рис. 4), сфероидов из опухолевых и нормальных клеток, полученных с помощью RGD-пептидов (рис. 5)).
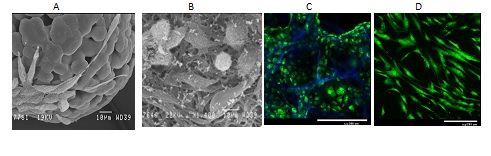
Рис.1. Рост мышиных фибробластов L929 на биодеградируемых полилактидных микроносителях (A), микроволокнах (B), в макропористых гидрогелях из хитозана и гиалуроновой кислоты (С), а также мезенхимальных стволовых клеток человека на хитозановых пленках, обработанных низкотемпературной плазмой в разряде постоянного тока (D). Клетки окрашены витальным красителем Сalcein AM (зеленый цвет), а структура матриксов визуализирована с помощью красителя DAPI (синий цвет). СЭМ (A,B) и конфокальная лазерная микроскопия (С,D).
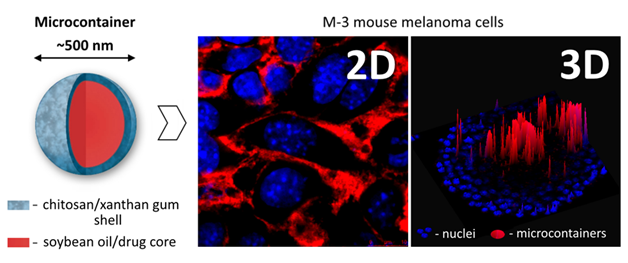
Рис. 2 Полисахаридные микроконтейнеры для доставки противораковых лекарств и их накопление в клетках M-3 (мышиная меланома) в 2D (монослойная культура) и в 3D (сфероиды) моделях.
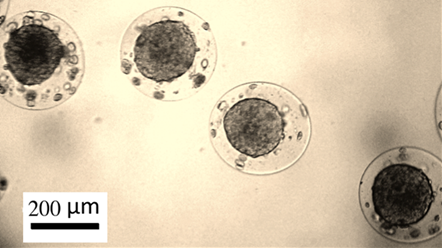
Рис.3. Опухолевые сфероиды из клеток рака молочной железы человека MCF-7, полученные культивированием в биосовместимых альгинат-хитозановых микрокапсулах.
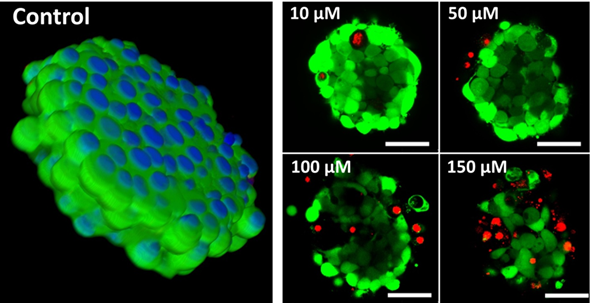
Рис. 4. Получение опухолевых сфероидов с помощью самопроизвольной агрегации клеток, индуцированной добавлением RGD-пептидов непосредственно к монослойным клеточным культурам.
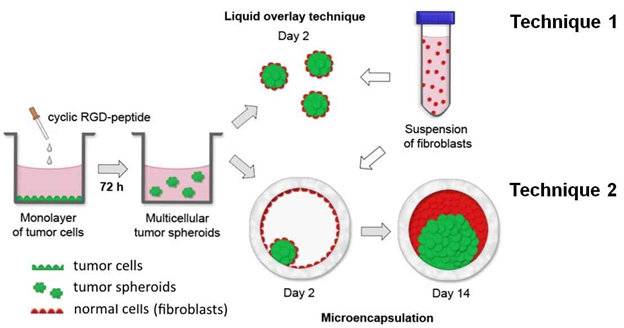
Рис.5. Два подхода для получения сфероидов из раковых и нормальных клеток c помощью RGD- пептидов.
| ФИО | Должность | Контакты |
|---|---|---|
| Марквичева Елена Арнольдовна, д.х.н. | г.н.с. | |
| Акасов Роман Александрович, к.х.н. | с.н.с. | |
| Селина Оксана Евгеньевна, к.х.н. | н.с. | |
| Гилёва Анастасия Михайловна | м.н.с. | |
| Дроздова Мария Георгиевна | н.с. | |
Ранее здесь работали | ||
| Хованкина Анна Викторовна | ||
| Бирюкова В.Н. | ||
| Мамедова А.Р. | ||
| Терехова В.В. | ||
| Смирнов И.В. | ||
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Марквичева Елена Арнольдовна
Москва, ул. Миклухо-Маклая, 16/10 — На карте
 Загрузка...
Загрузка...
