Центр биологических испытаний

|
Руководитель: Мурашев Аркадий Николаевич |
The Biological Testing Center is organized in the Department of Biological Testing of the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry to conduct the non-clinical studies on the OECD Principles of Good Laboratory Practice. It conducts Toxicity studies, Mutagenicity studies and Analytical and clinical chemistry testing.
Non-clinical studies, which are conducted at the Biological Testing Center:
- Acute Oral Toxicity (OECD TG 420, 423)
- Acute Dermal Toxicity (OECD TG 402)
- Acute Inhalation Toxicity (OECD TG 403, 433, 436)
- Repeated Dose 28-day Oral Toxicity Study in Rodents (OECD TG 407)
- Repeated Dose 90-Day Oral Toxicity Study in Rodents (OECD TG 408)
- Repeated Dose Dermal Toxicity: 21/28-day Study (OECD TG 410)
- Subchronic Dermal Toxicity: 90-day Study (OECD TG 411)
- Chronic Toxicity Studies in Rodents (OECD TG 452)
- Prenatal Development Toxicity Study (OECD TG 414)
- Reproduction / Developmental Toxicity Screening Test (OECD TG 421)
- Combined Repeated Dose Toxicity Study with the Reproduction / Developmental Toxicity Screening Test (OECD TG 422)
- Extended One-Generation Reproductive Toxicity Study (OECD TG 443)
- Carcinogenicity Studies (OECD TG 451)
- Combined Chronic Toxicity / Carcinogenicity Studies (OECD TG 453)
- In vivo Mammalian Erythrocyte Micronucleus Test (OECD TG 474)
- In vivo Mammalian alkaline comet assay (OECD TG 489)
- Toxicokinetics (OECD TG 417)
- Non-clinical safety studies for the conduct of human clinical trials for pharmaceuticals (ICH M3(R2))
- Safety pharmacology studies for human pharmaceuticals (ICH S7A)
- Chronic toxicity testing in rodents (ICH S4)
- Immunotoxicity studies for human pharmaceuticals (ICH S8)
- Detection of toxicity to reproduction for human pharmaceuticals (ICH S5(R3))
- Testing for carcinogenicity of pharmaceuticals (ICH S1B)
- Dose selection for carcinogenicity studies of pharmaceuticals (ICH S1C(R2))
- Genotoxicity testing and data interpretation for pharmaceuticals intended for human use (ICH S2 (R1))
The Biological Testing Center had the certificate of Good Laboratory Practice compliance from the Slovak National Accreditation Service since 2013 until 2023. The current certificate of Good Laboratory Practice compliance was received from TURKISH ACCREDITATION AGENCY. It is valid until 18.03.2028.
The Biological Testing Center has the certificate of Good Laboratory Practice compliance from the Russian Accreditation Service since 2014. The current certificate of Good Laboratory Practice compliance is valid until 2026.
The Biological Testing Center has been accredited by AAALAC (International Association for Assessment and Accreditation of Laboratory Animal Care) since 2005 until 2022.


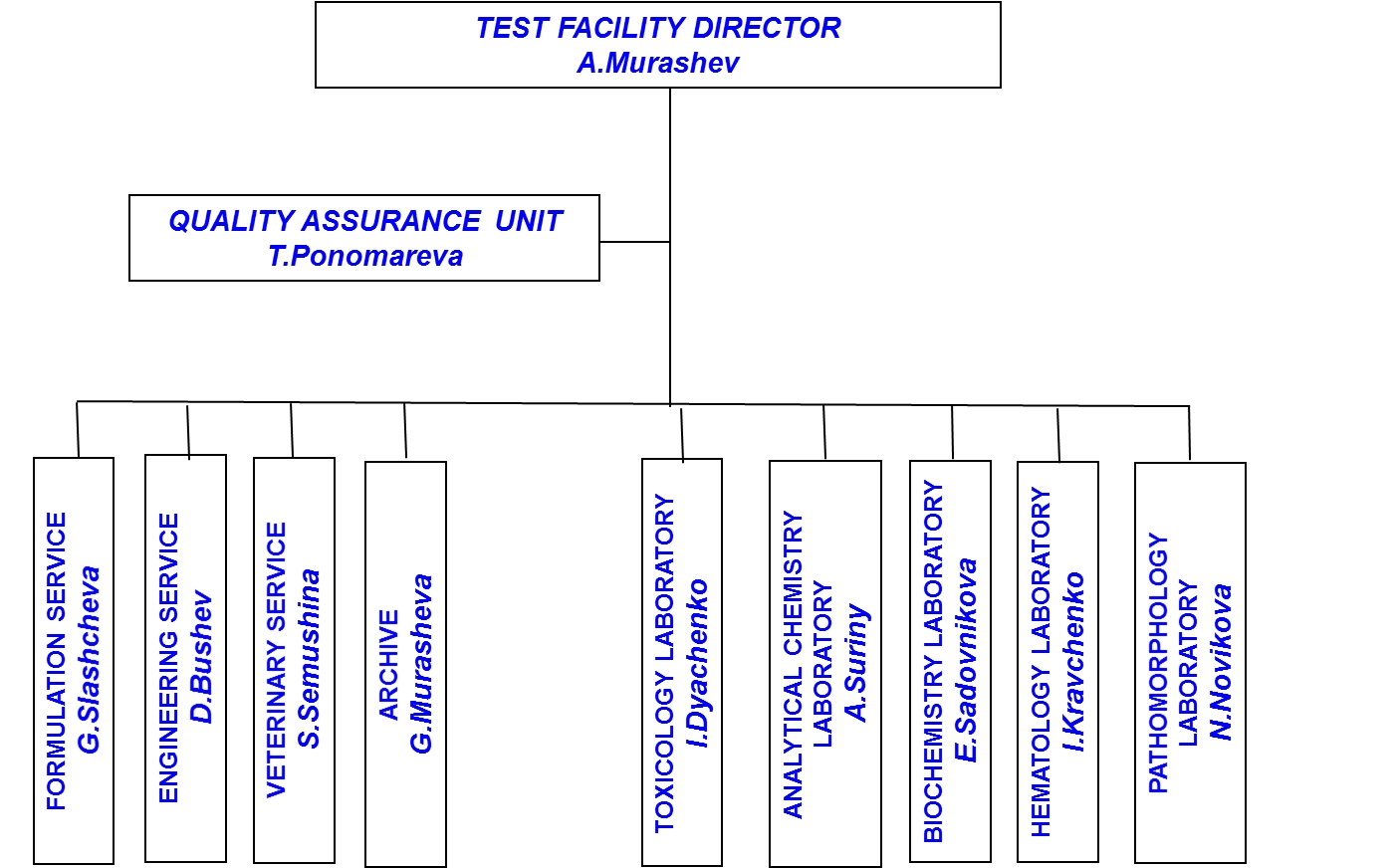
The Biological Testing Center has more than 50 employees
Biological Testing Center organizational chart
The Biological Testing Center is located in a building with a total area of about 3 thousand square meters with a vivarium (12 rooms of 16.5 square meters each) for keeping SPF-rodents. The animal rooms are supplied with 100% outside air (HEPA filtered) and are maintained at a positive pressure. Temperature (20-24oC) and humidity (40-60%) are controlled. 12-hour cycle of light is synchronized. Animals are given a standard chow and water ad libitum.
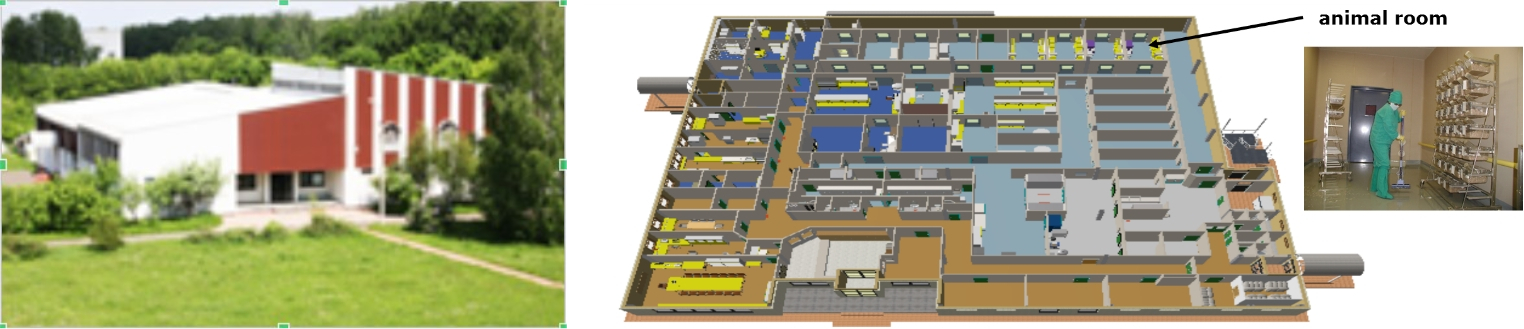
Building of Biological Testing Center and Layout chart of the test facility plan

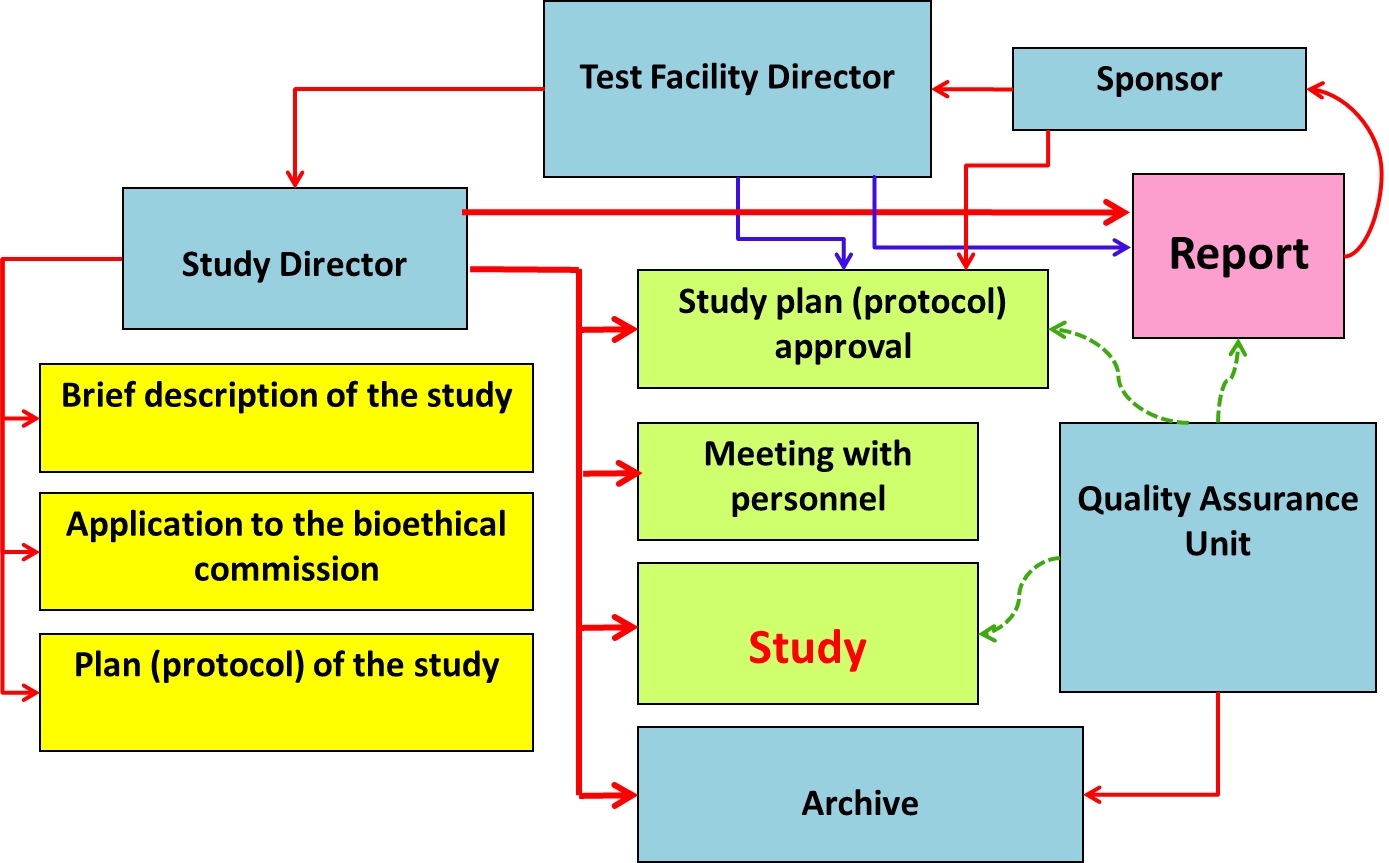
Non-clinical study organizational chart
The results of the non-clinical studies of the Biological Testing Center are recognized by the European Chemicals Agency
| Non-clinical studies, the results of which are published on the website of the European Chemicals Agency | ||
|---|---|---|
| CAS number | Non-clinical studies | OECD TG |
| 3077-27-8 | Combined Repeated Dose Toxicity Study with the Reproduction / Developmental Toxicity Screening Test | 422 |
| 2528-39-4 | Combined Repeated Dose Toxicity Study with the Reproduction / Developmental Toxicity Screening Test | 422 |
| 763-32-6 | Combined Repeated Dose Toxicity Study with the Reproduction / Developmental Toxicity Screening Test | 422 |
| 95-14-7 | Prenatal Development Toxicity Study | 414 |
| 29385-43-1 | Prenatal Development Toxicity Study | 414 |
| 78521-39-8 | Repeated Dose 90-Day Oral Toxicity Study in Rodents | 408 |
| 78521-39-8 | Prenatal Development Toxicity Study | 414 |
Мурашев Аркадий Николаевич
Пущино (Моск. обл.), проспект Науки, 6 — На карте
Scopus: 7004892371, ORCID: 0000-0002-2279-9285