Laboratory of proteolytic enzyme chemistry
The Laboratory of Proteolytic enzyme chemistry headed by RAS corresponding member RAS V.K. Antonov was founded in 1967 when it separated from the Laboratory of Antibiotic chemistry, led by academician M.M. Shemyakin. From 1992 Prof. L.D. Rumsh is the head of the Laboratory.
Presently, the laboratory performs structure and functional studies of Lon proteases, the research of regulatory roles of enteropeptidase and duodenase towards PAR and also the isolation and study of proteases of extremophilic organisms.
The laboratory participates in scientific and educational activity – three Dr.Sci. theses and more than 50 Ph.D. theses were prepared and defended, moreover, the considerable quantity of student master’s works was executed under the direction of the laboratory’s employees. The laboratory is the organizer of constantly operating all-Moscow Inter-Institute Seminar “Problems and perspectives in studies of proteolytic enzymes”, and also regularly organizes all-Russian symposiums “Chemistry of proteolytic enzymes”
The principal area of the laboratory’s research is the study of proteases which participate in regulation of intracellular proteolysis (ATP-dependent Lon proteases) and protein processing (enteropeptidase, duodenase). The extremophilic proteases are also among primary objects as well as proteases which are crucial for infection process of the diseases caused by such pathogens as Plasmodium falciparum and Human immunodeficiency virus.
The laboratory aims not only to study fundamental problems of modern enzymology (the determination of nature of protease selectivity towards their targets or the research of catalytic mechanism of certain enzymes), but also to work on applied problems (the isolation and study of proteases used in biotechnology and medicine, the search for inhibitors of various proteases and so on).
To investigate the catalytic mechanism of a series of proteolytic enzymes theoretical conformational analysis is successfully applied. An approach to distinguish general basic and covalent catalytic mechanisms of peptide hydrolysis using H218O as a reaction medium was developed. The catalytic mechanism of several proteases and peptidases was established. A peptide hydrolase classification based on the differences in their mechanisms was offered.
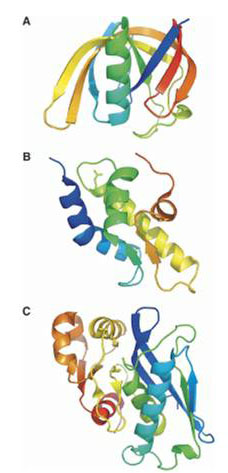
Of current interest and importance for retaining a cell proteome are ATP-dependent Lon proteases belonging to the AAA+ protein superfamily.
The proteolytic sites of Lon proteases was found to be represented by Ser-Lys catalytic dyad, and the Lon family to consist of two subfamilies (A and B) differing in arrangement of the fragments surrounding the active site residues, as well as in structural organization of the ATPase domains and a general molecule architecture. The comparative study of the Escherichia coli LonA (EcLonA) and Archaeoglobus fulgidus LonB (AfLonB) proteases revealed the importance of quaternary structure for the occurrence of proteolysis in a processive manner.
The X-ray analysis indicated that proteolytic domains of EcLonA and AfLonB posses the unique fold and form cyclic hexamers in crystals. The monomeric N-terminal subdomain of EcLonA also is of unique spatial structure while the organization of alpha-helical domain of Lons is typical for AAA+ proteins. The obtained results allowed designate Lon family as individual clan SJ in peptide hydrolyzes database MEROPS.
The study of gastrointestinal tract proteases resulted in isolation and characterization of novel enzyme – duodenase. The primary and three-dimensional structure of the enzyme were solved and the dual nature of its specificity (trypsin- and chimotrypsin-like) was discovered. The unique features of enteropeptidase from bovine duodenal mucosa possessing an absolute specificity were shown to be stipulated by a complex domain arrangement along with a strict hierarchy of the primary and two secondary substrate binding sites. One secondary substrate binding site provides high specificity while the other allows for high catalytic efficiency.
Data available indicate that duodenase can activate the proenteropeptidase, thereby inducing activation of the digestive proteolytic enzyme cascade. Moreover, the both enteropeptidase and duodenase also displayed a regulatory function relative to the protease activating receptors (PAR).
For the first time protease Ulysses from Drosophila virilis transposon (the aspartate HIV—1 protease homolog) was obtained, the enzyme characteristics were studied and its functional activity confirmed. The approaches for homologous modeling and molecular dynamics applied to design a three-dimensional structure of the protease Ulysses.
High-affinity inhibitors were obtained for the other aspartate protease –plasmepsin II from malarial parasite Plasmodium falciparum. The inhibitors were shown to possess expressive selectivity to plasmepsin II compared to human cathepsin D.
 Loading...
Loading...Scientific projects
 Loading...
Loading...Ivan Smirnov
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
Scopus: 7202245246, ResearcherID: N-5635-2017, ORCID: 0000-0002-0384-6568, Google Scholar
 Loading...
Loading...