Laboratory of molecular toxinology
The Laboratory carries out the fundamental studies on the molecular structures of animal venoms' components as well as molecular mechanisms of action of animal venoms and their components. Toxins are used as the main instrument for investigation of mechanisms of the nervous system function and of the nerve impulse transduction, in particular. As a result of expansion of interests and tasks of the Laboratory, the studies of the venoms which affect the hemostasis have been initiated in recent years.
The Laboratory of molecular toxinology cooperates with several foreign groups and laboratories. Thus, the study of various receptors and search for new toxins are carried out jointly with Ruhr University Bochum (Germany, Ruhr-Universität Bochum). Besides, the toxins are studied in cooperation with Belarusian scientists (Institute of Physiology, National Academy of Sciences of Belarus), who studied the effect of neuroactive compounds from venoms on the development of tumors (Ehrlich carcinoma is used as a tumor model). Of particular interest is the cooperation with the specialists from Vietnam (The Institute of Applied Materials Science, Ho Chi Minh City), research on toxins of Vietnamese animals is carried out together with the Vietnamese scientists. Also the Laboratory of molecular toxinology has good relations with the Indian scientists.
In addition to the traditionally studied neurotoxins currently the Laboratory explores the peptides and proteins that affect blood clotting. Scientists are involved in the study of their structure and the determination of biological properties. In addition, the staff of the Laboratory, together with colleagues from the branch of Institute of Bioorganic Chemistry (Pushchino) investigates hypotensive peptides which have been found in the venom of a Burmese viper Azemiops feae. The Department of molecular bases of neurosignaling carries out preclinical studies on the peptide of this poisonous snake which interacts with the cholinergic receptors and is positioned as a local muscle relaxant. This is the reason why in the future it can be used for treatment of a number of diseases, where the local muscle relaxation is necessary.
The Laboratory has been organized in 2009 and together with the Laboratory of ligand-receptor interactions constitutes the Department of molecular bases of neurosignaling. The complementary methods of structural neurobiology allow the laboratories to carry out investigations in close cooperation.
- Conducting the fundamental studies on the molecular structures of animal venoms' components as well as molecular mechanisms of action of animal venoms and their components.
- Exploring the peptides and proteins that affect blood clotting, studying their structure and the determination of biological properties.
As a result, more than three dozen of proteins with new structural and functional properties were isolated and characterized recently.
Thus, several new toxins were found during the study of Naja kaouthis cobra venom. In particular, for the first time a glycosylated three-fingered toxin – cytotoxin 3 from Naja kaouthia venom was found. So far it is the only glycosylated three-fingered toxin. It was demonstrated that glycosylation resulted in substantial decrease of toxin cytotoxicity. We have shown that so called weak toxins which were well know for many years are capable to interact with nicotinic acetylcholine receptors. Although acting only at micromolar concentrations, weak toxins have an advantage of being practically nontoxic while producing a long lasting effect. Moreover these toxins allosterically interact with muscarinic acetylcholine receptors. A disulfide bound dimers of three-fingered toxins were also found in the Naja kaouthia venom for the first time. It was shown that such a dimerization results in the change of biological activity of toxins forming dimer.
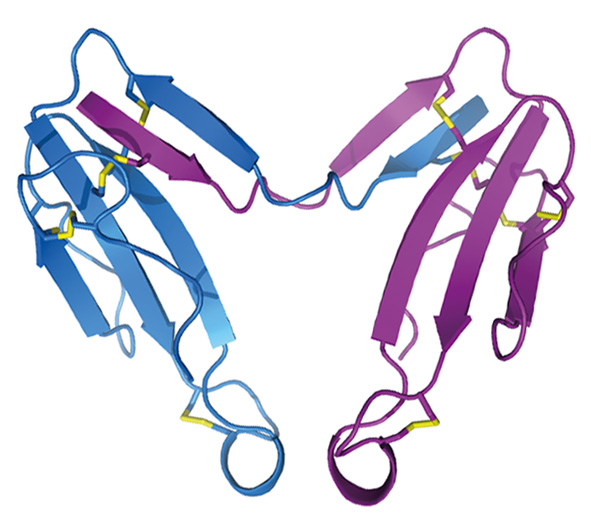
Crystal structure of alpha-cobratoxin dimer (Osipov et al., (2012) J. Biol. Chem. 287(9), 6725-6734.)
- Several neurotoxic proteins were isolated from viper venoms. Thus two heterodimeric phospholipases A2 possessing presynaptic activity were isolated from Nikolski’s viper venom. From puff adder Bitis arietans venom, we isolated and characterized the novel protein bitanarin that reversibly blocks nAChRs. Bitanarin is the first described phospholipase A2 that contains 14 disulfide bonds within a single polypeptide chain, and thus it represents a new structural type of phospholipases A2 by both the length of polypeptide chain and the number of cysteine residues. Bitanarin possesses the neurotoxic properties unique for phospholipases A2: it binds to nicotinic acetylcholine receptors and blocks acetylcholine-elicited current. On the other hand, bitaranin is the first competitive acetylcholine receptor blocker possessing phospholipase A2 activity.
- Several new proteins affecting hemostasis were also isolated from snake venoms and characterized. Thus, a new metalloproteinase oxiagin was isolated from Naja oxiana cobra venom. Oxiagin was found to inhibit the classical pathway of the complement system by preventing the formation of C3-convertase. To achieve it, oxiagin binds to IgG on the surface of sheep erythrocytes sensitized with rabbit antibodies, thus, preventing the interaction of component C2 (without its inactivation) with immobilized C4b. A new anticoagulant phospholipase A2 have been isolated from the venom of the Egyptian cobra Naja haje. The protein retards fibrin clot formation by inhibiting thrombin. This protein is the first thrombin inhibitor found in the venoms of the Elapidae family and a first example of new structural type of polypeptide thrombin inhibitors.
| Fullname | Position | Contacts |
|---|---|---|
| Yuri Utkin, D.Sc | Head of lab., pr. r. f. | utkin@ibch.ru |
| Igor Dyachenko, D.Sc | s. r. f. | |
| Aleksej Osipov, Ph.D. | s. r. f. | |
| Elvira Shaykhutdinova, Ph.D. | s. r. f. | |
| Starkov V.G. | r. f. | |
| Alina Ismailova | res. eng. | |
| Maria Severyukhina | res. eng. | |
| Suhov D.A. | res. eng. | |
| Mahtumova M. | eng. | |
| Timofeev N.D. | eng. | |
Previously worked here | ||
| Irina Shelukhina, D.Sc | shelukhina.iv@yandex.ru | |
| Averin A.S. | ||
| Andrei Siniavin, Ph.D. | ||
| Natalya Borozdina | ||
| Kost V.Y. | ||
| Viktor Palikov | ||
| Yuliya Palikova | ||
| Ramazanova A.S. | ||
| Andreeva T.V. | ||
| Kazakov O.V. | ||
| Rashimova A.D. | ||
| Gorbacheva E.I. | ||
| Afanas'eva S.O. | ||
| Epifanova L.A. | ||
| Garifulina A.I. | ||
| Mescheryakov P.A. | ||
| Spirova E.N., Ph.D. | ||
 Loading...
Loading...Scientific projects
 Loading...
Loading...Yuri Utkin
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
31/606
Scopus: 35598587900, ORCID: 0000-0002-4609-970X, ResearcherID: F-1958-2014, Google Scholar
 Loading...
Loading...
