Лаборатория углеводов
Лаборатория занимается синтезом олигосахаридов и гликоконъюгатов, а также самоассоциирующих пептидов и гликопептидов, дизайном гликочипа, углевод-белковыми взаимодействиями, лектинами млекопитающих и бактерий, естественными антителами к углеводам.
Мультиантигенный микрочип
Сотрудники Лаборатории совместно с Consortium for Functional Glycomics разработали гликановый микроэррей (printed glycan array, PGA) – мультиантигенный микрочип, который благодаря наличию в нем опухоле-специфических маркеров (гликанов) дает возможность проведения прогностики, диагностики и мониторинга онкологических заболеваний на новом уровне. Чип используется как в фундаментальных исследованиях, так и при разработке диагностических подходов, с целью диагностики ряда онкологических и репродуктивных заболеваний.

Супрамолекулярная химия
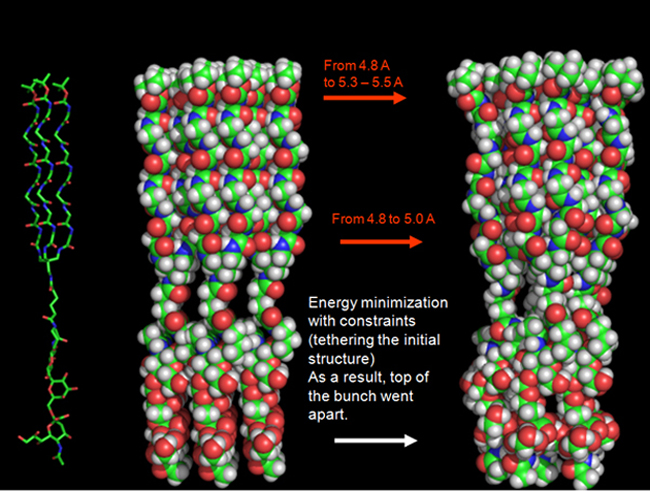
Интерес к самосборке малых молекул обусловлен возможностью дизайна наноматериалов с индивидуальными свойствами и молекулярных устройств. Для сборки нано-материалов и устройств используются простые молекулы, состоящие почти исключительно из олигоглициновых цепей, когда несколько таких цепей образуют star-like молекулу, супрамеры собираются благодаря водородным связям. В супрамерах типа (Glycosyl-S)n, S-фрагмент обеспечивает сборку, а углеводная часть обеспечивает биологическую активность и водорастворимость. Подробности здесь.
Синтез олигосахаридов и гликоконъюгатов
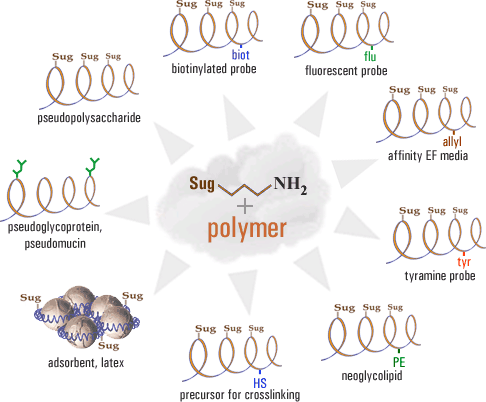
Лаборатория занимается синтезом олигосахаридов и гликоконъюгатов – универсальных инструментов для различных биохимических и иммунологических исследований, таких, как изучение специфичности лектинов, разработка ингибиторов адгезии вируса гриппа и др. Подробности здесь и здесь.
Модификация клеточной поверхности
Лаборатория занимается изучением переноса гликолипидов между клетками, а также разработкой синтетических гликолипидов (и аналогично построенных липофильных пептидов), способных встраиваться практически в любые клетки. Модификация этими молекулами эритроцитов позволяет проводить выявление антител самым простым из возможных методов – агглютинацией. Среди терапевтических подходов в первую очередь необходимо отметить онкотерапию, основанную на инициации иммунного противоопухолевого ответа: инъекция в опухоль гликолипида приводит к немедленной иммунной атаке предсуществующими естественными антителами, направленными к гликану данного гликолипида (дайте, пожалуйста, ту же ссылку, что в англ. версии).
Выявлен ряд природных антител, которые специфически связываются и разрушают клетки рака молочной железы. Подробности здесь.
Кроме того, Лаборатория занимается разработкой вакцин на основе дендритных клеток.
Лаборатория сотрудничает с подразделениями Института, а также с ФГБУ «Российский онкологический научный центр им. Н. Н. Блохина» Минздрава России, ФГБУ «Научный центр акушерства, гинекологии и перинатологии имени академика В.И. Кулакова» Минздрава России, Клиника Белвидже каталонского Института здоровья (Барселона, Испания), Институт исследования здоровья Университета Нанта (Франция), Оклендский технологический университет (Новая Зеландия), Институт Гликомики Университета Гриффита (Австралия), Базельский университет (Швейцария), Масариков университет (Чехия) и рядом других университетов.
Лаборатория (сначала Группа) Углеводов была организована в конце 1988 года, она стала естественным продолжением направления, заложенного в Институте академиком Н.К. Кочетковым, а затем (после его ухода директором в ИОХ) проф. А.Я. Хорлиным («Лаборатория Гликопротеинов и смешанных биополимеров»).
- Синтез олигосахаридов и гликоконъюгатов, самоассоциирующих пептидов и гликопептидов;
- Дизайн диагностического гликочипа;
- Изучение углевод-белковых взаимодействий;
- Естественные антитела к углеводам, В1-клеточный иммунитет.
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Бовин Николай Владимирович
Москва, ул. Миклухо-Маклая, 16/10 — На карте
Scopus: 7103341757, ORCID: 0000-0001-8669-4477
- Факс: +7 (495) 330-55-92
 Загрузка...
Загрузка...
