Press-room / news / Science news /
Genetically-encoded BRET-activated photodynamic therapy for the treatment of deep-seated tumors
Being targeted to tumor cells, genetically-encoded NanoLuc-miniSOG construct generates internal light source and sensitizer pair, which makes possible PDT effect under BRET activation to treat tumors at virtually unlimited depth.
Photodynamic therapy (PDT) is one of the most appealing photonic modalities for cancer treatment based on anticancer activity of light-induced photosensitizer-mediated reactive oxygen species (ROS), but a limited depth of light penetration into tissues does not make possible the treatment of deep-seated neoplasms and thus complicates its widespread clinical adoption. Here, scientists from the Department of immunology and the Department of Peptide and Protein Technologies IBCh RAS in collaboration with colleagues from Nursery for laboratory animals, GPI RAS and MEPhI introduce the concept of genetically-encoded bioluminescence resonance energy transfer (BRET)-activated PDT, which combines an internal light source and a photosensitizer (PS) in a single genetic construct, which can be delivered to tumors seated at virtually unlimited depth and then triggered by the injection of a substrate to initiate their treatment. To illustrate the concept, we engineered genetic NanoLuc-miniSOG BRET pair, combining NanoLuc luciferase flashlight and phototoxic flavoprotein miniSOG, which generates ROS under luciferase substrate injection. We prove the concept feasibility in mice bearing NanoLuc-miniSOG expressing tumor, followed by its elimination under the luciferase substrate administration. Then, we demonstrate a targeted delivery of NanoLuc-miniSOG gene, via tumor-specific lentiviral particles, into a tumor, followed by its successful elimination, with tumor growth inhibition (TGI) coefficient exceeding 67%, which confirms a great therapeutic potential of the proposed concept. In conclusion, this study provides proof-of-concept for deep tissue “photodynamic” therapy without external light source that can be considered as an alternative for traditional PDT.
The results are published in the Light: Science & Applications.
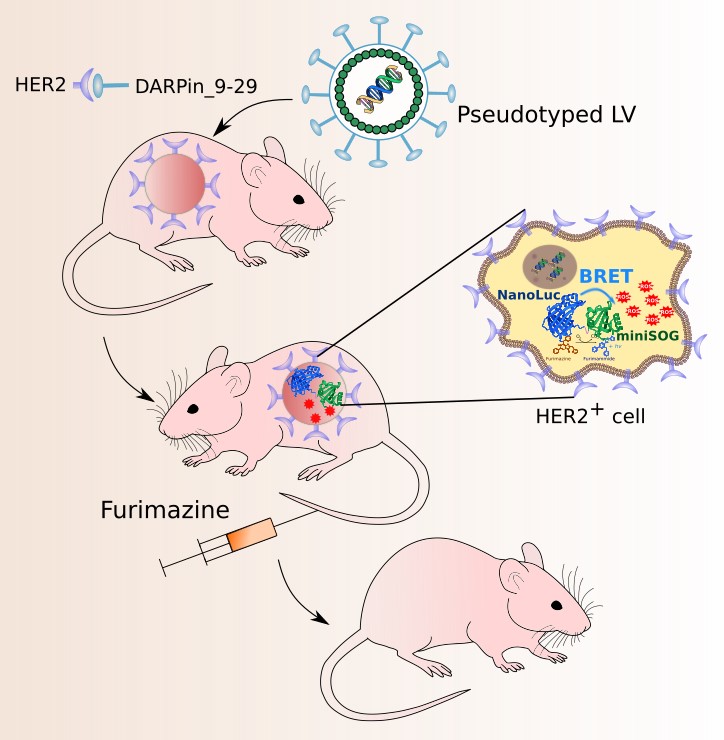
Targeted PDT using HER2-specific lentiviruses (LVs) as carriers of NanoLuc-miniSOG gene. HER2-positive tumor is targeted with HER2-specific LVs carrying the NanoLuc-miniSOG gene. DARPin_9-29 incorporated in LV’s envelope targets LVs to HER2-specific tumor. NanoLuc luciferase is fused to miniSOG phototoxic protein at genetic level. The energy generated by luciferase substrate furimazine upon its oxidation by NanoLuc is nonradiatively transferred to miniSOG resulting in its excitation and generation of ROS, which in turn kill cancer cells
march 16, 2022