Press-room / news / Science news /
Synthesis of 2-chloropurine ribosides with chiral amino acid amides at C6 and their evaluation as A1 adenosine receptor agonists
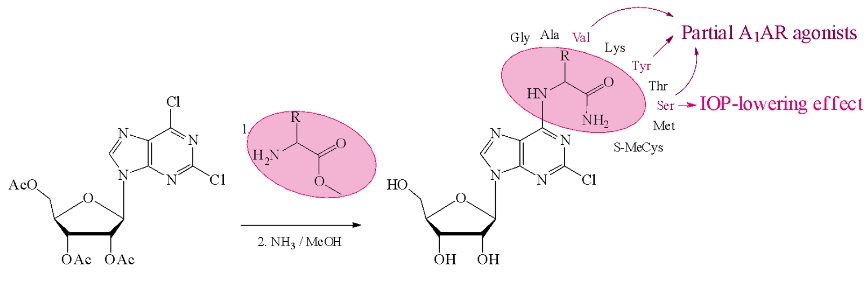
The team of scientists from the Department of biotechnology and the Laboratory of structural biology of ion channels IBCh RAS synthesized a series of adenosine analogs as purine ribonucleosides bearing amino acid amides at the C6 position of 2-chloropurine.
Molecular docking by affinity for A1 adenosine receptors (A1ARs) was conducted. Colleagues from Volgograd State Medical University studied the A1AR stimulating activity in a model of an isolated mouse atrium. The investigation of A1AR stimulating activity of synthesized nucleosides was carried out in a model of an isolated mouse atrium. Derivatives with tyrosine, valine, and serine residues exhibit the properties of A1AR partial agonists. Animal experiments in the open field test have shown that these compounds have different profiles of psychoactive action. Serine derivative has intraocular pressure-lowering effect. The synthesized nucleosides can be the basis for further design and synthesis of new A1AR agonists. The results are published in the Bioorganic Chemistry.
june 14, 2022


