Press-room / news / Science news /
Synthesis of New 5ʹ-Norcarbocyclic Aza/Deaza Purine Fleximers - Noncompetitive Inhibitors of E.coli Purine Nucleoside Phosphorylase
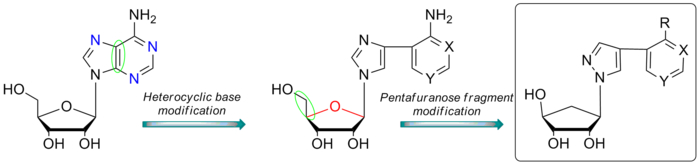
Scientists from the Laboratory of biosynthesis of physiologically active compounds and Laboratory of biopharmaceutical technologies (IBCH RAS) and Engelhardt Institute of Molecular Biology of the Russian Academy of Sciences synthesized a new series of flexible 5′-norcarbocyclic aza/deaza-purine nucleoside analogs and evaluated as potential inhibitors of E. coli purine nucleoside phosphorylase.
A series of flexible 5′-norcarbocyclic aza/deaza-purine nucleoside analogs were synthesized from 6-oxybicyclo[3.1.0.]hex-2-ene and pyrazole-containing fleximer analogs of heterocyclic bases using the Trost procedure. The compounds were evaluated as potential inhibitors of E. coli purine nucleoside phosphorylase. Three analogs were found to be noncompetitive inhibitors with inhibition constants of 14–24mM. From the data obtained, it can be assumed that the new 5′-norcarbocyclic nucleoside analogs interact with the active site of the PNP like natural heterocyclic bases. But at the same time the presence of a cyclopentyl moiety with 2′ and 3′ hydroxyls is necessary for the inhibitory properties, since compounds without those groups did not exhibit an inhibitory effect under the experimental conditions.
The results are published in the Frontiers in Chemistry (IF 5.545).
july 11, 2022


