Press-room / news / Science news /
Mechanism of Translation Termination Revealed
Protein biosynthesis by the ribosome is one of the most important reactions in nature. The length of the polypeptide chain is strictly determined by the start and stop codons in mRNA. Translation termination is necessary for the timely completion of protein biosynthesis and the release of the polypeptide product from the ribosome. This process is regulated by specific proteins, the so-called "release factors", which recognize stop codons and mediate the hydrolysis of the ester bond in the peptidyl-tRNA molecule localized in the catalytic center of the ribosome. The new study shows the role of the release factor and the 2'-OH group of tRNA in the catalysis of polypeptide cleavage through the formation of a "bridged" cyclic intermediate. The work was published in the journal Science.
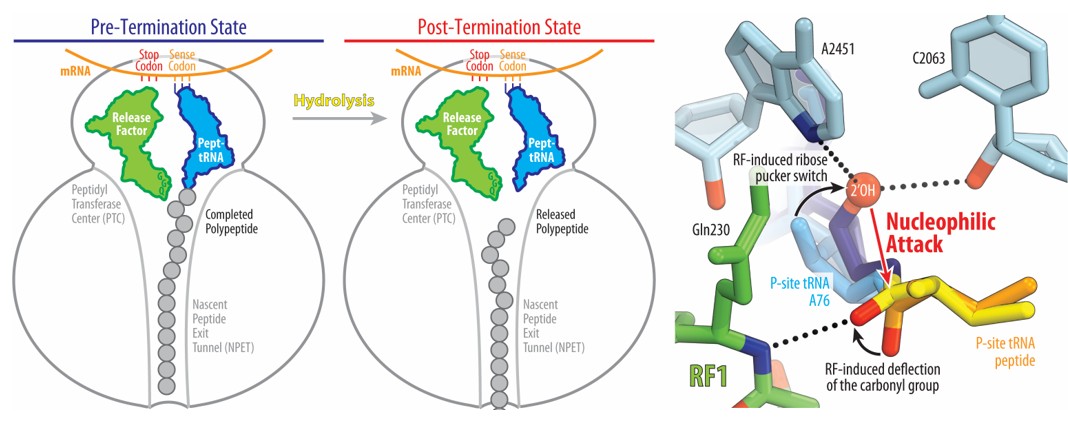
Figure. The process of translation termination is shown schematically on the left: the release factor recognizes the stop codon and induces hydrolysis of the peptidyl-tRNA. On the right, a fragment of the complex structure in the pre-termination state is shown. The release factor (RF1) forms a hydrogen bond (dashed line) with the carbonyl of the peptidyl-tRNA and induces a change in the ribose pucker. The 2'-OH group of the 3'-terminal nucleotide of tRNA is positioned for nucleophilic attack onto the carbonyl of the C-terminal amino acid of the peptide.
The prevailing view in the field was that the release factor activates a water molecule that performs hydrolysis of the ester bond of the peptidyl-tRNA. The preparation of stable derivatives of peptidyl-tRNA in which the ester bond is replaced by an amide bond allowed the study of high-resolution crystal structures of complexes between the ribosome, mRNA, release factor, and peptidyl-tRNA in the P-site, corresponding to the "prehydrolysis" state (see Figure). Surprisingly, no water molecule activated by the release factor was detected. Instead, it turned out that a hydrogen bond is formed between the amide nitrogen of the main chain of the conserved motif of the release factor and the carbonyl of the peptidyl- tRNA. This results in:
- a change in the ribose pucker from C2'-endo to C3'-endo, positioning the 2'-OH group for nucleophilic attack on the carbonyl carbon of the C-terminal amino acid;
- stabilization of the resulting "bridged" cyclic intermediate.
Apparently, the polypeptide chain migrates to the 2'-position, and only then does hydrolysis occur.
Thus, new structural data have suggested a mechanism of translation termination that is fundamentally different from what was previously assumed. The role of the release factor is not in direct activation of a water molecule, but in inducing a specific conformation of the 3'-terminal nucleotide of tRNA, thereby promoting the activity of its own 2'-hydroxyl, which mediates hydrolysis. Such a conformation is not realized during translation elongation, excluding the possibility of premature termination.
The work was supervised by Prof. Yury Polikanov (University of Illinois at Chicago) and Prof. M.G. Gagnon (University of Texas at Galveston). The authors dedicated their work to the memory of their mentors: Prof. Thomas Steitz, Nobel Prize laureate for the study of the structure and function of the ribosome, and Prof. Alexander Spirin, leading scholar in the study of protein biosynthesis in Russia.
june 27, 2025


