Press-room / news / Science news /
Biotechnologies based on the fungal bioluminescence pathway
The journal Trends in Biotechnology published a review devoted to biotechnologies based on the fungal bioluminescence pathway (FBP). Unlike luminescent reporter systems that require the addition of a substrate, FBP uses the natural metabolite caffeic acid to maintain continuous, autonomous luminescence. This allows for the production of autonomously luminescent living organisms and overcomes the key limitations of previous technologies. The review examines recent advances in the creation of luminescent plants and FBP-based biosensors and discusses the potential applications of this reporter system in science and the economy.
An international team of scientists, including researchers from the Institute of Bioorganic Chemistry, Russian Academy of Sciences, published a review in the journal Trends in Biotechnology. The review summarizes the current state and scientific context of the development of autoluminescent organisms using the fungal bioluminescence pathway. Bioluminescence is widespread in nature (in bacteria, fungi, insects, and marine organisms) and has long been used as a basis for creating reporter tools for research. The main components of bioluminescent systems are luciferase enzymes and luciferin substrates. For most bioluminescent systems, the mechanisms of substrate biosynthesis are unknown, requiring their external addition. This complicates continuous visualization of processes in living systems. FBP is a metabolic cycle in which a series of enzymatic reactions leads to the continuous generation of light. FBP is based on the conversion of a common natural metabolite, caffeic acid, to hispidin, which is converted to 3-hydroxyhispidin, a fungal luciferin. Oxidation of fungal luciferin leads to the formation of oxyluciferin and the generation of light. Oxyluciferin is then recycled into caffeic acid.
The study of FBP began with the identification of the genes encoding the bioluminescent system of the fungus Neonothopanus nambi in 2018, which enabled the transfer of this pathway to other organisms. In particular, it became possible to achieve fully autonomous luminescence in transgenic plants. The first luminescent plants produced only a limited amount of light, so engineering enzymes and metabolic pathways were required to create new generations of luminescent plants. For example, the addition of the enzyme NpgA, required for post-translational modification of the enzyme hispidin synthase (nnHispS), significantly increased light output in plants, mammals, and yeast. At the same time, metabolic engineering of the caffeic acid biosynthetic pathway was conducted: the introduction of the plant enzyme 4-coumaroylshikimate 3'-hydroxylase increased the level of caffeic acid biosynthesis, which increased the brightness of model tobacco plants by approximately 10-fold. Another approach involved suppressing competing metabolic pathways using artificial microRNAs and introducing additional tyrosine metabolism genes (tyrosine ammonia lyase from Rhodotorula glutinis and 4-hydroxyphenylacetate 3-monooxygenase from bacteria), which led to an even greater increase in bioluminescence.
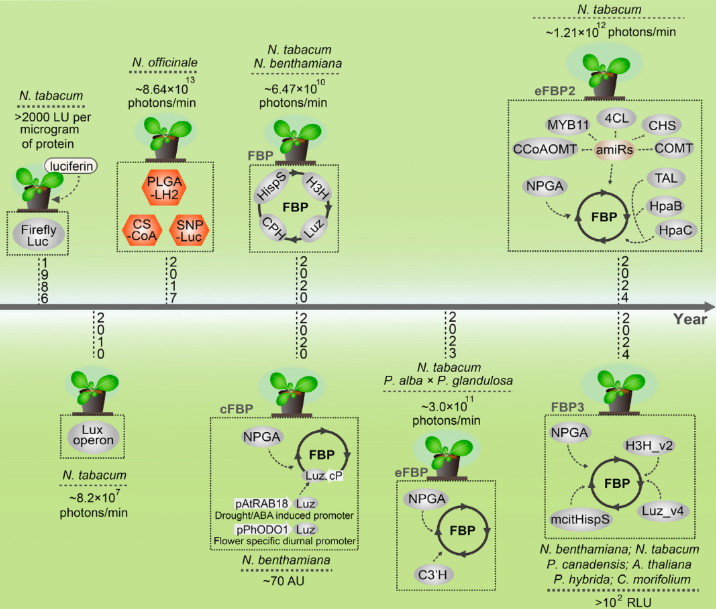
In addition to optimizing the metabolic pathway, significant attention was paid to improving the FBP enzymes. Directed evolution was used to create new variants of the nnLuz luciferase and nnH3H enzyme. The nnLuz_v4 luciferase contains seven amino acid substitutions, provides more intense luminescence and is stable at elevated temperatures. Analogs of the nnHispS enzyme in other bioluminescent fungi were also searched for: using the mcitHispS enzyme from the fungus Mycena citricolor resulted in increased brightness compared to the original enzyme from Neonothopanus. Incorporation of NpgA and improved variants of nnLuz and nnH3H created a new version of the FBP2 pathway, while the integration of mcitHispS created FBP3. FBP3 is characterized by an increase in bioluminescence brightness by more than two orders of magnitude compared to the original FBP1 system. Importantly, introducing these genes did not negatively impact plant growth and development, and the resulting luminescence can be recorded using a smartphone camera. Through comprehensive optimization, autoluminescent plants have achieved a level of luminescence that can be observed without specialized equipment. This is a key step toward practical application.
The emergence of autonomously luminous organisms opens up a wide range of applications, such as the use of plants for illumination (planterns). Theoretical estimates indicate that a tree with a canopy of approximately 30 m^2 would need to devote only ~0.3% of its energy production to bioluminescence to emit light comparable in brightness to streetlights. Thus, even a small fraction of a plant's photosynthetic energy can be converted into visible luminescence. Partially replacing electric lights with bioluminescent trees and other plants has the potential to reduce energy consumption and associated CO2 emissions. In 2024, a glowing petunia created using the FBP system was commercialized. The first batch of these plants quickly sold out, demonstrating the high public interest in luminous ornamental plants. The development of luminous garden plants and trees could lead to the creation of parks and walkways illuminated by living plants.

FBP-based technologies enable the study of gene expression dynamics and protein-protein interactions, drug screening, and high-throughput screening. An important area of research is biosensor plants, which alter the intensity or pattern of their luminescence in response to specific environmental stimuli. For example, using stress-oriented promoters that control luciferase gene, plants were created that glow during drought (monitoring increased levels of abscisic acid, pRAB18) or during pathogen attack (via signals from salicylic acid (pWRKY70) and jasmonic acid (pORCA3)). Such biosensor plants enable non-invasive and real-time monitoring of plant physiological status. In the future, such FBP-based sensor systems could form the basis of "smart" agricultural technologies, alerting farmers to the onset of drought, nutrient deficiency, or crop disease long before visible symptoms appear.
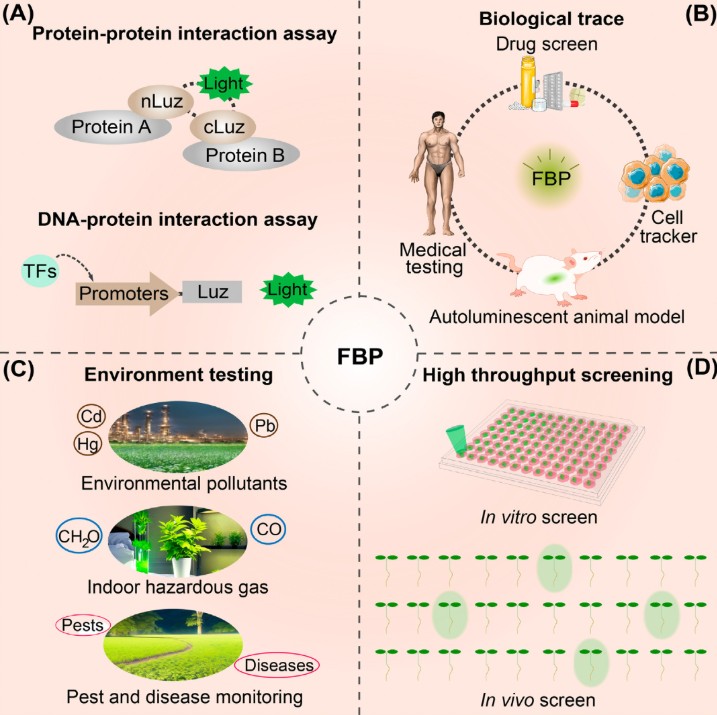
Despite progress, there are still problematic aspects of FBP that need to be addressed. Current luminescence brightness in mammalian cells remains low, due to the low catalytic activity of the key enzymes—hispidin synthase HispS and luciferase Luz. Precise brightness control is also currently impossible, which may not always be optimal in terms of energy consumption and ecology. This problem could be partially addressed by using inducible promoters that would activate or enhance luminescence only when needed (for example, at night or under certain conditions). Furthermore, the uncontrolled spread of the FBP transgene into natural ecosystems must be prevented. Proposed measures include the creation of sterile plant lines, the use of chloroplast transformation (genes are introduced into the plastid genome and are not transmitted via pollen), and the manipulation of flowering time.
In conclusion, the review emphasizes that recent advances in artificial intelligence (AI) and synthetic biology will improve key characteristics of FBP—spectral range, brightness, and stability. AI-based approaches trained on large biological datasets enable rapid prediction of mutation effects and de novo design of improved enzymes, thereby accelerating and simplifying protein engineering. For example, AI-guided directed evolution facilitates efficient optimization of enzyme activity, while generative models support the design of luciferases with specified functions. Beyond the engineering of individual enzymes, AI facilitates systemic optimization of metabolic pathways, such as FBP, by predicting enzyme stoichiometry, identifying metabolic bottlenecks, and improving the balance of metabolic fluxes. Integrated AI platforms further accelerate the biodesign pipeline—from designing genetic circuits to predicting the characteristics of entire organisms.
november 10, 2025


