Laboratory of organic synthesis
The laboratory conducts molecular design, the development of synthetic schemes and synthesis a wide variety of classes of compounds in preparative amounts. For example, tri- and tetrasaccharide receptors of the SiaLex tetrasaccharide, substituted arylboronic acids of varying acidity, indol derivatives, 7-hydroxycoumarin, pyrrole-2,3-dicarboxylic acid, di- and tri-carboxylic acids of thiazole derivatives, oligoglycine fragments with N-benzylglycine, 1,2-dihydroquinoline-3-carboxylic acid, derivatives of bicyclic complex.
The laboratory conducts experiments to search for the possible conditions of abiotic synthesis of adenosine and adenosine monophosphate, as well as work on the properties, synthesis and possible use of ionic liquids - a fundamentally new type of solvents.
Moreover researchers are engaged in the creation of fluorescent probes for DNA, anesthetics and muscle relaxants new generation, as well as developing methods for the determination of drugs.
Laboratory cooperates with laboratories of the Institute, The Vernadsky Institute of Geochemistry and Analytical Chemistry of the Russian Academу of Sciences, The Faculty of Chemistry of the Moscow State University, The Engelhardt Institute of Molecular Biology of the Russian Academy of Sciences, The Bach Institute of Biochemistry of the Russian Academy of Sciences, The Mendeleev University of Chemical Technology of Russia, etc.
The Laboratory was founded on the basis of organic synthesis Group (worked since 1994) in 2010.
• Development of innovative methods of synthetic organic chemistry and innovative schemes of synthesis of organic compounds
• A practical preparation of compounds of different classes, such as benzoheterocycles, arylsulfamides and arylsulfonic acids, polycyclic and cage compounds, arylboronic acids, drug degradation products, antigens and haptens, fluorescent labels, unconventional amino acids, spin-labeled amino acids of the chromophore and photosensitizing compounds, low-molecular weight compounds.
• Oligopeptide components of Plm II protease inhibitors – ω-hydroxy-L-α-amino acids – were synthesized.
• A new preparative method for the synthesis argiope, synthesized 100 g of this natural toxin. Founded method was used in the synthesis of argiope analogues.
• Efficient methods for synthesis were designed and representative set of substituted 2-thiazolyl-1-alkyl(aryl)ethanol, prospective templates for new pharmacological agents.
• Synthetic schemes were developed and synthesis of haptens were conducted, which play an important role in an enzyme immunoassay toxic components of household detergents that pollute the environment, modern pesticides and antibiotics.
• The methods of synthesis were designed and photoswitchable protein fragment to create a controlled secondary structure elements was received; photoactivated labels for studies of receptor systems; fluorescent labels for the study of structure and functions of nucleic acids; photosensitizing agents; fluorescent indicators.
• As a part of the Program of the Presidium of the RAS "Biosphere Origin and Evolution" were analyzed the abiogenous variations of the adenine formation conditions and condensation of adenine with ribose and ribosophosphate using the methods of computer synthesis; monosaccharides library were formed, methods of determining the methods of gas chromatography-mass spectrometry and GLC were developed.
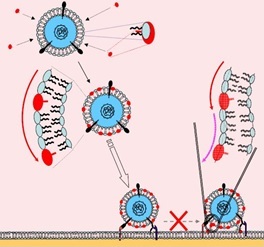
• New subclass of modified nucleosides having high activity against enveloped viruses was founded. Peruse detected earlier (Bioorgan. Chemistry, 29 (3), 289-294 (2003), Nucleosides, Nucleotides & Nucleic Acids, 24 (5/7), 923-926 (2005), Tetrahedron, 62 (6), 1279 -1287 (2006) Problems of Virology, 2006 (1), 34-38, Org. Biomol. Chem., 4 (6), 1091-1096 (2006)) identified a class of antiviral compounds from the group of substances active against enveloped viruses. Specifically, IC50 5- (perylene-3-yl) ethynyl-2'-deoxyuridine was 50 nmol / L for herpes simplex virus type 1 and 180 nmol / L for hepatitis C. A characteristic feature of active nucleosides structure is the availability of an aromatic residue hydrocarbon perylene rigidly attached to the nucleobase via a triple bond. It is suggested that the unique structure of the resulting compounds is responsible for their incorporation into the virion membrane, resulting in the fusion of the virion to the cell (infection) becomes very disadvantageous process (Figure). The novel compounds practically cytotoxic.
| Fullname | Position | Contacts |
|---|
 Loading...
Loading...Scientific projects
 Loading...
Loading...Formanovsky A.A.
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
 Loading...
Loading...
