Лаборатория химии гетероциклических соединений
Основным направлением исследований группы является разработка новых подходов к синтезу гетероциклических соединений, а также применение этих подходов с синтезе веществ обладающих биологической активностью или являющихся моделями в изучении биологических процессов.
Основная деятельность группы направлена на разработку новых и усовершенствование старых подходов к синтезу гетероциклических соединений, а также прикладное использование полученных методов в синтезе целевых соединений. В качестве последних выступают:
- Модельные соединения, имитирующие хромофоры флуоресцентных белков
- Новые флуоресцентные красители
- Новые флуорогенные красители
- Прочие биологически активные соединения
Группа химии гетероциклических соединений образовалась в коллективе ученых вышедших из Лаборатории молекулярных технологий ИБХ РАН, возглавляемой академиком Сергеем Анатольевичем Лукьяновым. Долгое время наш коллектив был частью лаборатории Биофотоники, а также группы природных соединений. В роли самостоятельного подразделения наше группа начала функционировать еще в 2015 году, однако только в 2017 году было принято решение о выделении ее в отдельное подразделение ИБХ.
Изучение хромофоров флуоресцентных белков
Одним из основных направлений наших исследований является изучение строения хромофоров окрашенных и флуоресцентных белков. Ключевым инструментом таких исследований, безусловно, является встречный синтез. Ранее этот подход позволил подтвердить строение хромофоров белков asFP595, Kaede и zFP538, а также разобраться в механизме формирования хромофора белка dsRed.
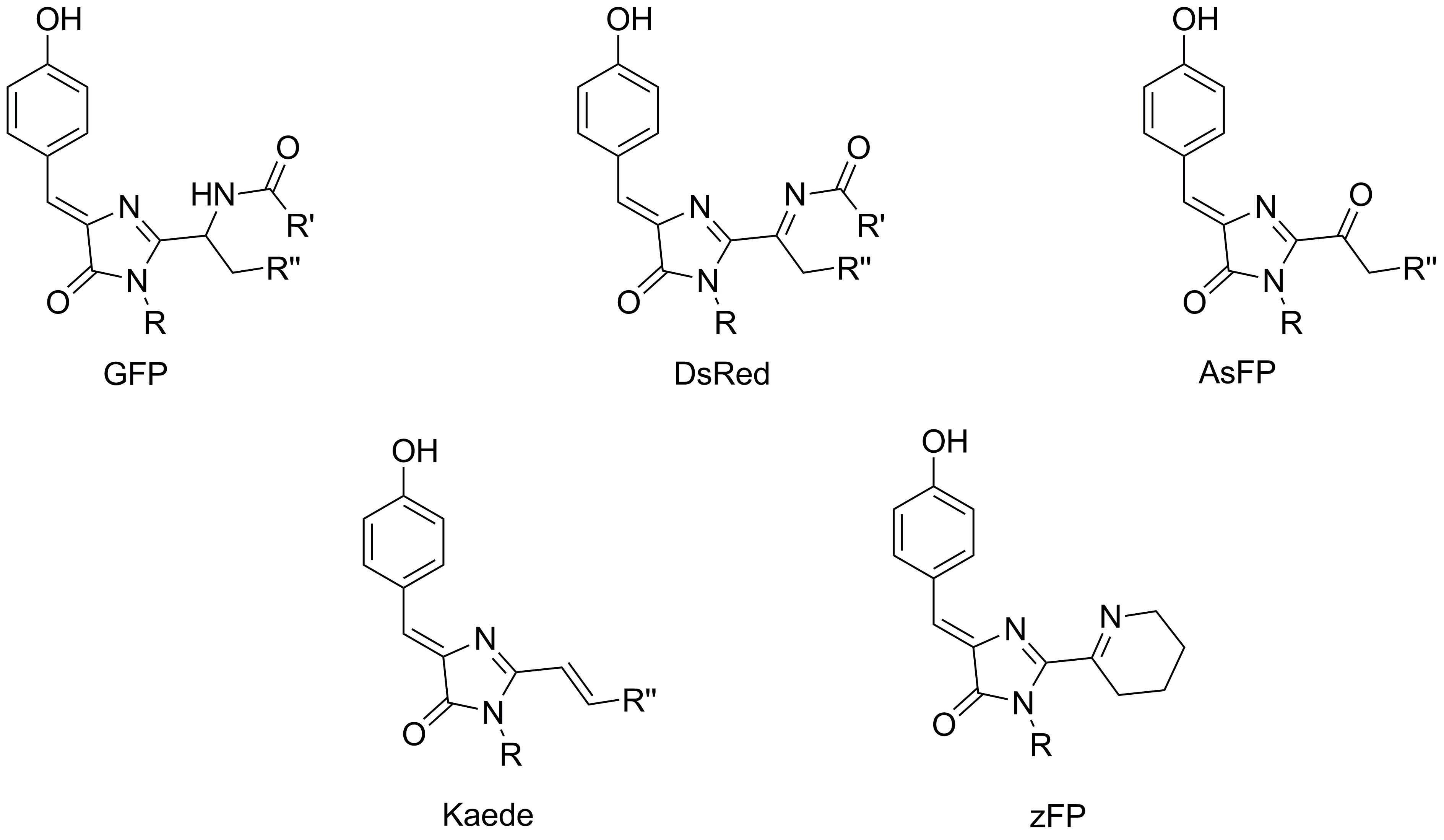
В настоящее время в рамках проекта 16-33-60116-мол-а-дк ("Изучение хромофоров флуоресцентных белков: от структурно-функциональных исследований к поиску новых флуорофоров для живых систем") нами также была подтверждена структура хромофоров желтых и оранжевых белков, содержащих остаток триптофана, а также начата работа по синтезу модельного соединения, имитирующего структуру хромофора белка laRFP.
Изучение нового класса флуоресцентных красителей на основе борированного хромофора GFP
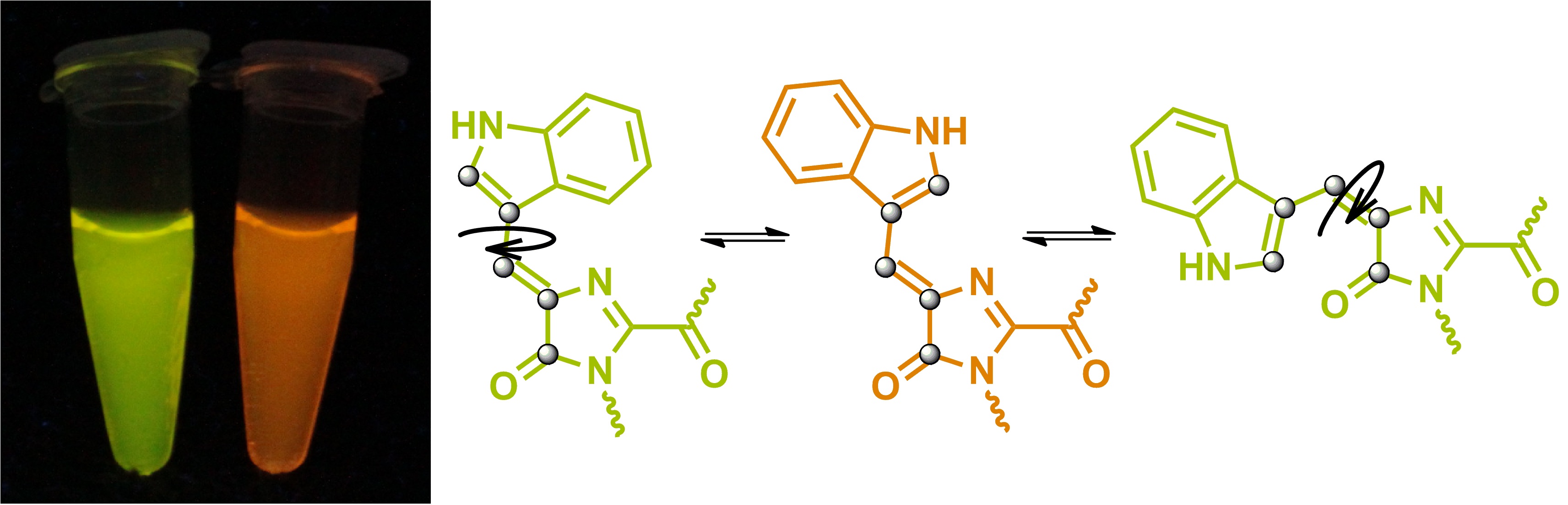
Еще одним интересным результатом наших исследований стало выявление зависимости квантового выхода флуоресценции хромофоров от их подвижности, которое помогло нам синтезировать ряд высокофлуоресцентных производных хромофора GFP с помощью координационной фиксации атомом бора, которые можно с уверенностью отнести к новой отдельной группе флуоресцентных маркеров получивших название BOBDI (от английского BOronBenzyliDeneImidazolone). Это открытие позволило на практике показать возможность использования таких соединений в роли флуоресцентных меток для живых систем (работа реализована в рамках проекта РФФИ 14-03-31162 мол_а, «Новый класс флуоресцентных красителей для биологии»).
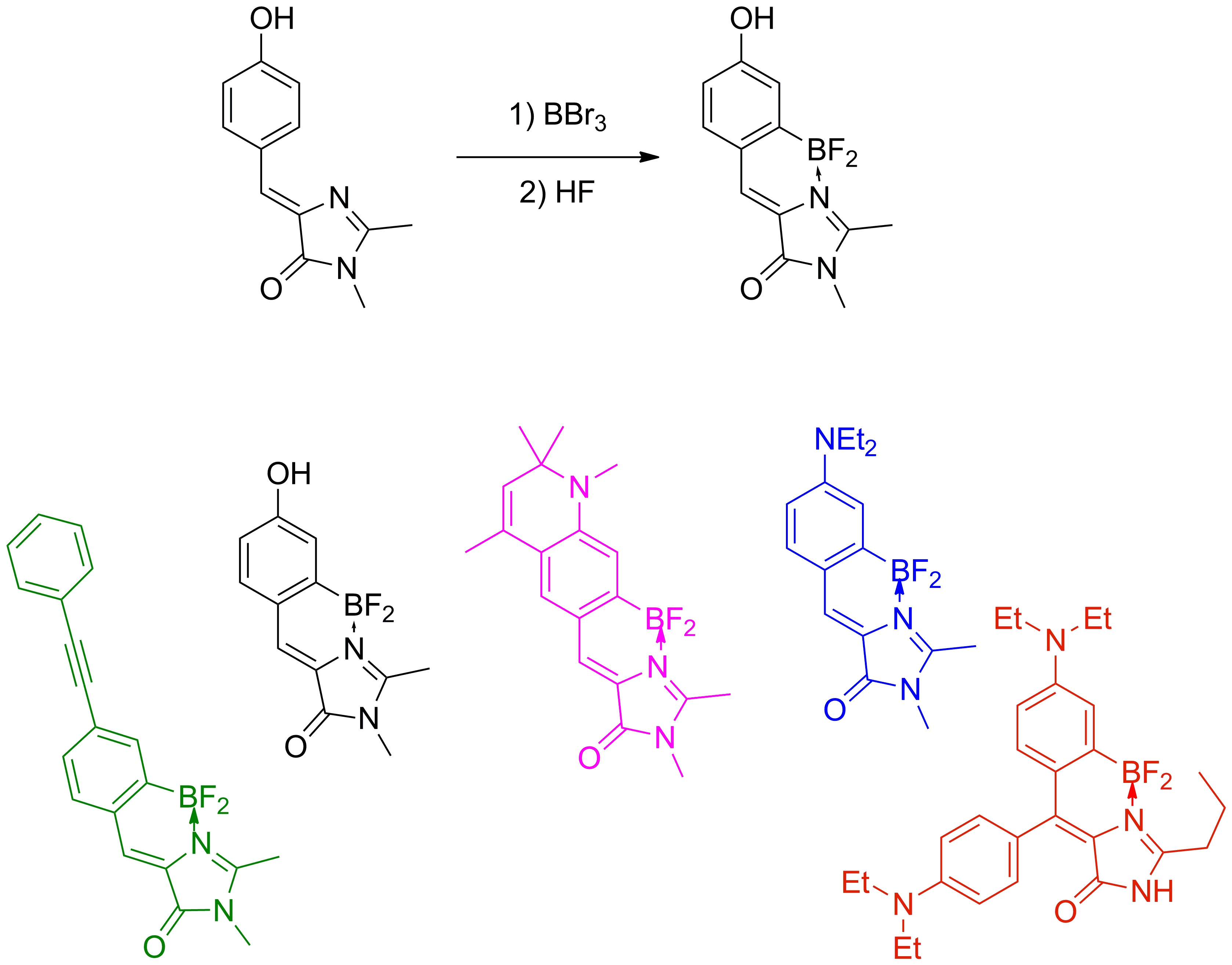
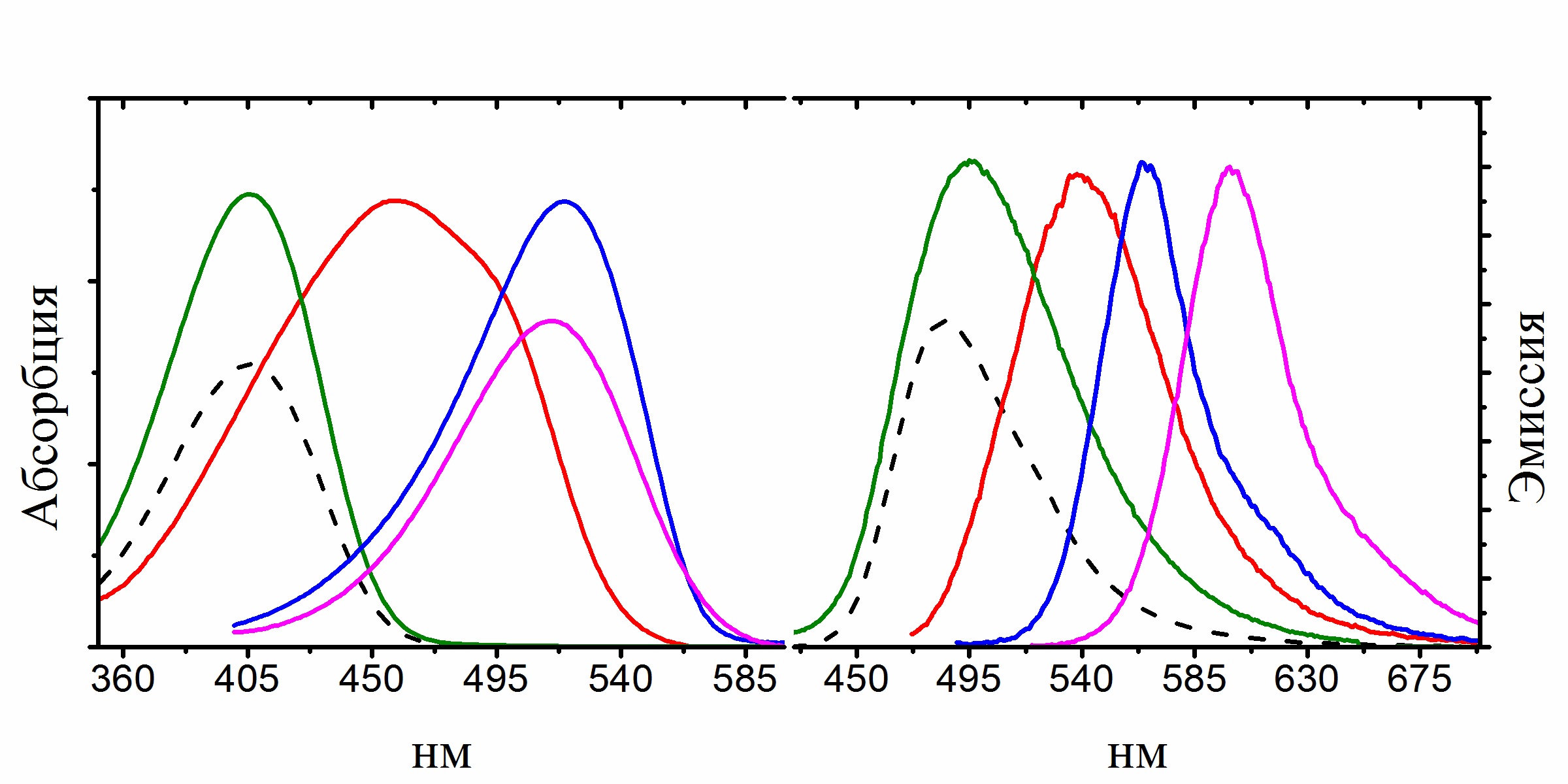
Существующие флуоресцентные красители, используемые для окрашивания живых систем, обладают рядом существенных недостатков и непригодны для решения определенного круга задач (например, среди них практически нет соединений, обладающих Стоксовыми сдвигами более 100 нм). В то же время хромофоры флуоресцентных белков лишены многих недостатков присущих существующим красителя, а потому они являются отличной базой для создания новых красок.
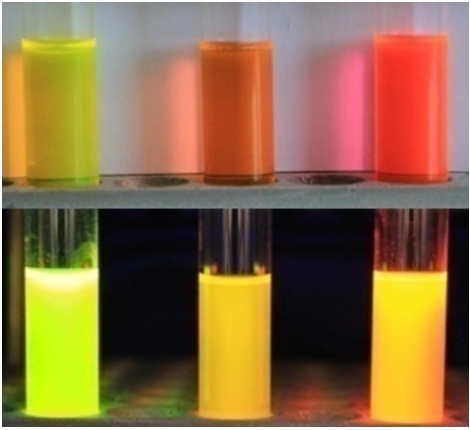
Разработка новых флуорогенные красителей, в том числе на основе хромофоров флуоресцентных белков
Одним из новых и современных методов флуоресцентному мечению биологических объектов является использование так называемых флуорогенных красителей – веществ, которые не имеют выраженной флуоресценции в свободном виде и приобретают ее лишь при связывании с целевым объектом.
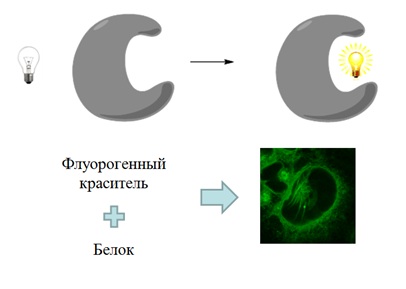
Одним из перспективных кандидатов на роль подобных веществ являются хромофоры флуоресцентных белков и их производные.
В связи с этим в нашей группе активно ведется создание и изучение различных флуорогенных соединений.
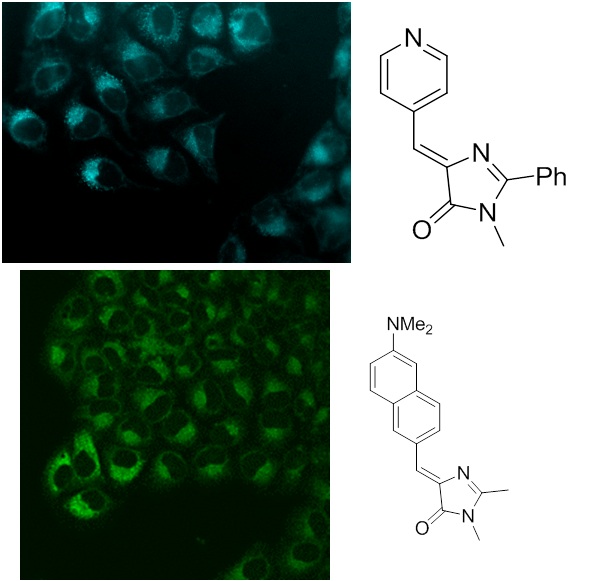
Разработка новых подходов к синтезу гетероциклических соединений
Наш коллектив долгое время занимался изучением химии хромофоров флуоресцентных белков имеющих в своей основе молекулу – 4-бензилиден-имидазол-5-онов. За время этой работы нами было создано несколько новых подходов к синтезу этих соединений, а параллельно было открыто множество неожиданных превращений связанных с использованием эфиров нитроуксусной и азидоуксусной кислот.
В частности, обнаруженный нами метод синтеза 5-гидрокси-1,2-оксазин-6-онов позволяет по-новому взглянуть на одну из методик синтеза производных изоксазол-3,5-дикарбоновых кислот – реакцию Дорнова.
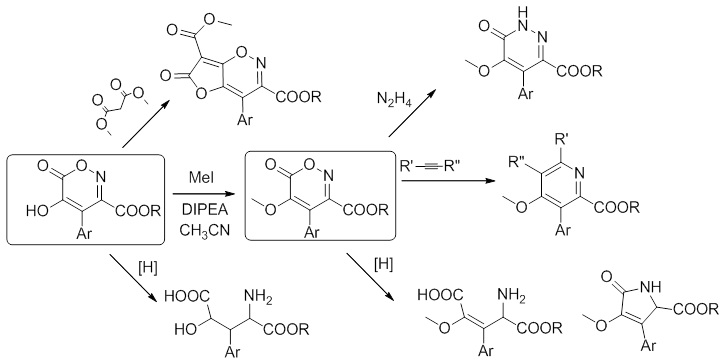
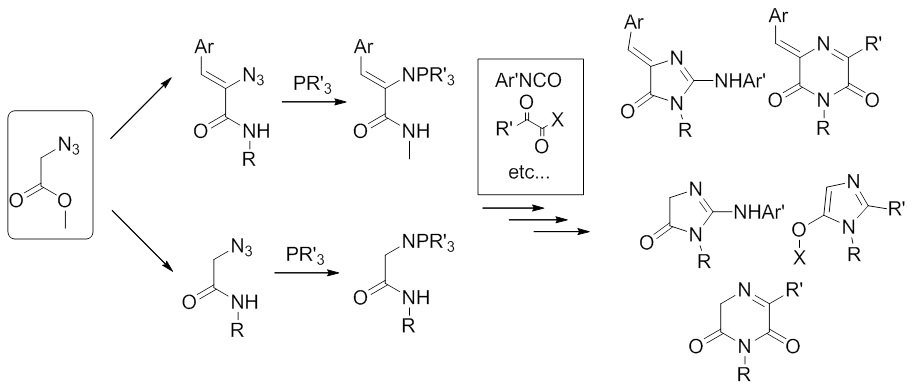
Аналогично, наблюдаемые нами превращения производных азидоуксусной кислоты и их фосфазенов, также не находят отражения в научной литературе, что позволяет предположить возможность создания новых путей синтеза гетероциклических систем и из этих реагентов.
2012-2014 Разработан ряд новых подходов к синтезу производных хромофоров флуоресцентных белков. Изучение свойств полученных соединений позволило пролить свет на причину низкого квантового выхода флуоресценции свободных хромофоров.
(Baranov M.S. et al // J. Am. Chem. Soc. 2012. #134(13). 6025–6032; Baranov M.S., et al. // Chem. Comm. 2013. #49. 5778-5780)
2013-н.в. Получено новое семейство флуоресцентных красителей – борированных производных этих хромофоров. Показана возможность использования этих соединений в роли флуоресцентных маркеров используемых для окрашивания биологических объектов.
(Baranov M.S. et al // Chemistry - A European Journal. 2014. #20(41), 13234–13241; Frizler M. et al // Org. Biomol. Chem. 2013. #11. 5913-5921; Baleeva N.S. et al // Eur. J. Org. Chem. 2015. #26. 5716-5721; Baleeva N.S. et al // Tetrahedron Lett. 2016, 3043-3045.)
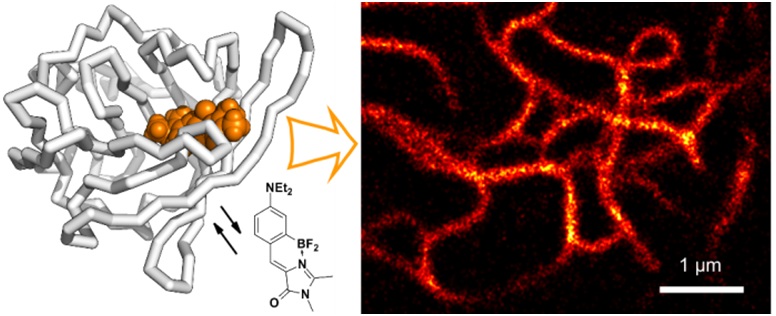
2013-н.в. Показана возможность использования производных хромофоров флуоресцентных белков в роли флуорогенных красителей (веществ, чья флуоресценция проявляется особенно ярко при связывании с целевыми объектами) для визуализации в живых объектах, в том числе с применением флуоресцентной спектроскопии сверхвысокого разрешения.
(Povarova N.V. et al // J. Mater. Chem. C. 2016. #4. 3036-3040)
2012-н.в. Открыто нескольких новых методов синтеза и модификации гетероциклических соединений, включающих оксазолы, оксазины, а также дикетопиперазины - аналоги лекарственных средств против вируса гриппа.
(Baranov M.S., et al // Chemistry of Heterocyclic Compounds 2012. #48(7). 1108–1110; Baranov M.S. et al // Tetrahedron Lett. 2013. #54. 628–629; Baranov M.S., et al // Synth Comm. 2013. #17. 2337-2342; Baranov M.S., et al // Tetrahedron. 2014. #70(23). 3714-3719; Golodukhina S.V. et al // Chemistry of Heterocyclic Compounds 2015. #51(10). 944–947)
| ФИО | Должность | Контакты |
|---|---|---|
| Баранов Михаил Сергеевич, д.х.н. | в.н.с. | baranovmikes@gmail.com |
| Богданова Юлия Антоновна, к.б.н. | н.с. | |
| Гильванов Айдар Римович | м.н.с. | |
| Мяснянко Иван Николаевич, к.х.н. | м.н.с. | |
| Смирнов Александр Юрьевич, к.х.н. | м.н.с. | |
| Иванов Дмитрий Сергеевич | тех.-лаб. | |
| Краснова С.А. | тех.-лаб. | |
| Люкманова Г.Р. | тех.-лаб. | |
| Молчанова Марина Витальевна | тех.-лаб. | |
| Мустафин Д.А. | инженер | |
| Опрышко В.Е. | инженер | |
| Рудик Даниил Игоревич | инженер | |
| Фрадков А.Ф., к.х.н. | инженер | |
Ранее здесь работали | ||
| Балеева Надежда Сергеевна, к.х.н. | ||
| Зайцева Эльвира Романовна | ||
| Низовцев Алексей Вадимович, к.х.н. | ||
| Соколов Анатолий Игоревич | ||
| Зайцева С. | ||
| Алексеева И.А. | ||
| Тюшина И.В. | ||
| Юнисова Г.Р. | ||
| Жигилева Екатерина Андреевна | ||
| Николаев С.Е. | ||
| Соколинская Елена Леонидовна, к.б.н. | elena.sokolinskaya@gmail.com | |
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Баранов Михаил Сергеевич
Москва, ул. Миклухо-Маклая, 16/10 — На карте
ORCID: 0000-0002-9339-7603, Scopus: 55170275100, ResearcherID: L-5014-2016
34/421
 Загрузка...
Загрузка...
