Laboratory of chemistry of heterocyclic compounds
The main direction of the group's research is the development of new approaches to the synthesis of heterocyclic compounds, as well as the application of these approaches in the synthesis of substances which have biological activity or are models in the study of biological processes.
The main activity of the group is aimed at the development of new and improvement of old approaches to the synthesis of heterocyclic compounds, as well as the applied use of the methods obtained in the synthesis of the target compounds. The next ones act in the capacity of the target compounds:
- Model compounds which simulate chromophores of fluorescent proteins
- New fluorescent dyes
- New fluorogenic dyes
- Other biologically active compounds
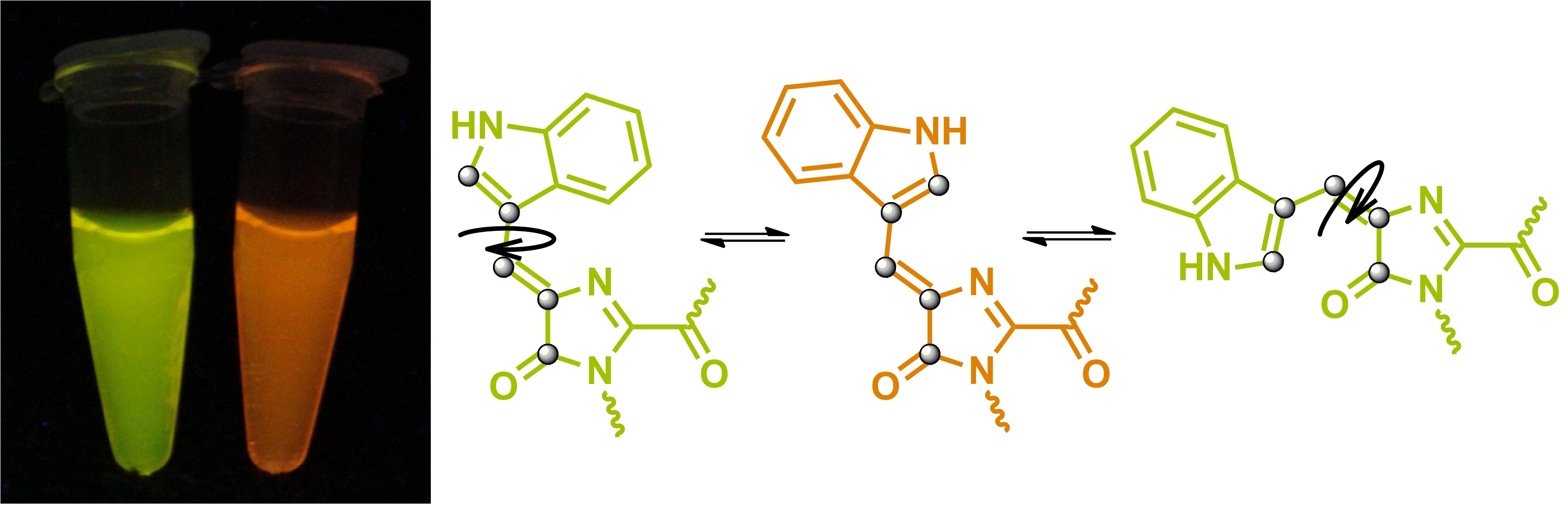
One of the main directions of our research is the study of the structure of stained and fluorescent proteins chromophores. Counter-synthesis is certainly the key tool of such studies. Earlier this approach allowed to confirm the structure of chromophores of proteins asFP595, Kaede and zFP538, as well as to understand the mechanism of dsRed protein chromophore formation.
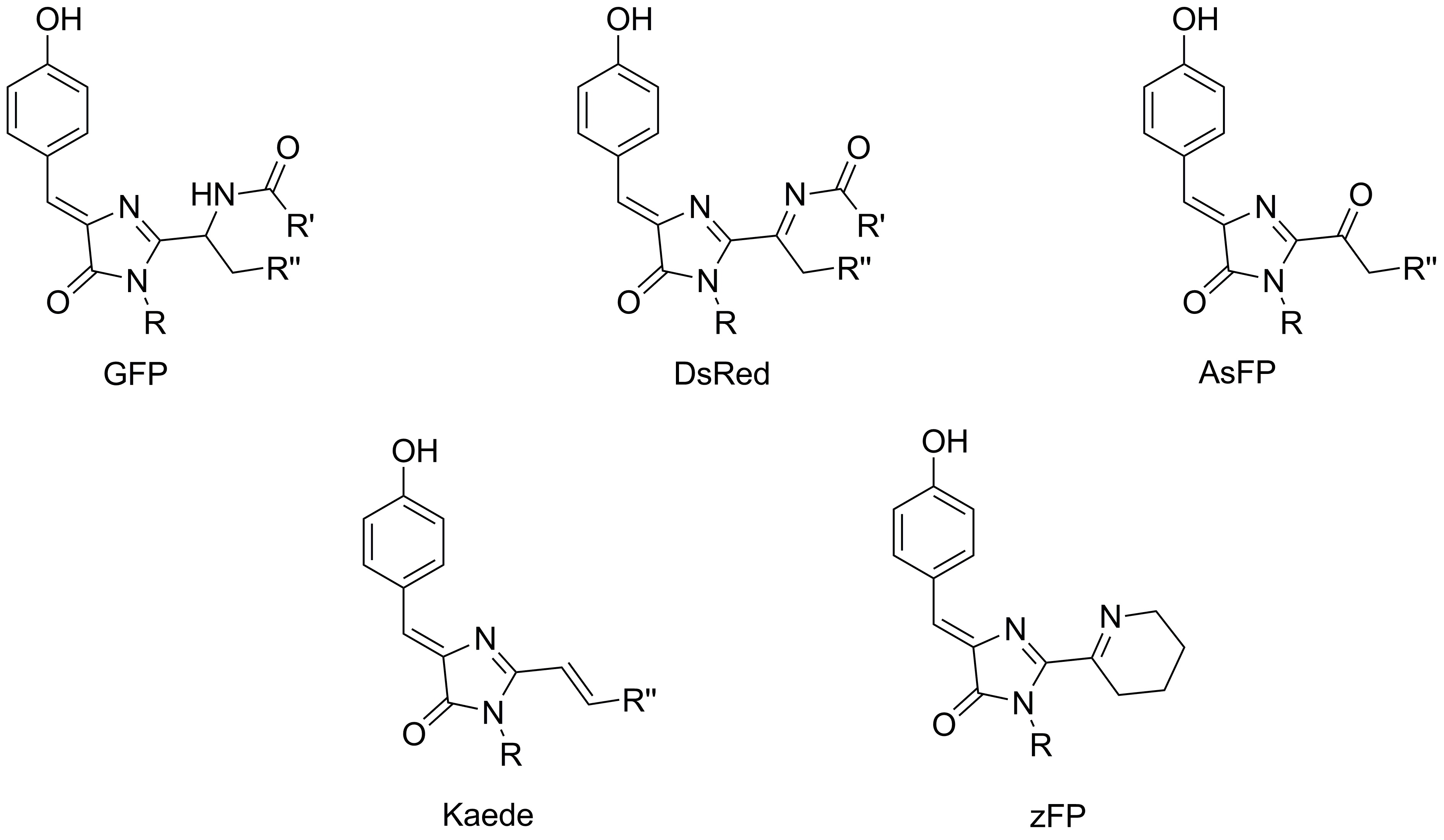
Currently, within the framework of project 16-33-60116-mol-a-dk ("Study of the chromophores of fluorescent proteins: from structural and functional studies to the search for new fluorophores for living systems"), we also confirmed the structure of the chromophores of yellow and orange proteins, which contain the residue tryptophan, and also work on the synthesis of a model compound, which simulate the chromophore structure of the laRFP protein was started.
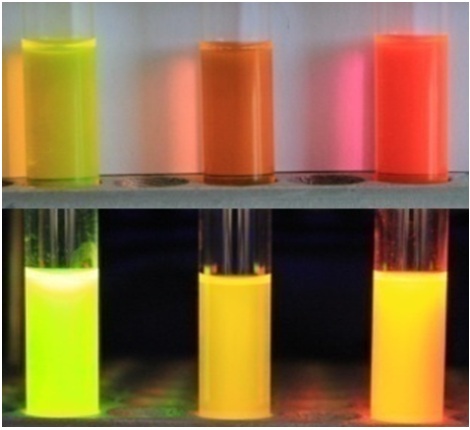
Study of a new class of fluorescent dyes based on the borated chromophore GFP
Another interesting result of our studies was the detection of the dependence of the quantum yield of fluorescence of chromophores on their mobility, which helped us synthesize a number of high fluorescence derivatives of the GFP chromophore by means of coordination fixation with a boron atom, which ones can be reliably attributed to a new separate group of fluorescent markers called BOBDI BOronBenzyliDeneImidazolone). This discovery made it possible in practice to demonstrate the possibility of using such compounds as fluorescent labels for living systems (the work was realized within the framework of the RFBR project 14-03-31162 mol_a, "A new class of fluorescent dyes for biology").
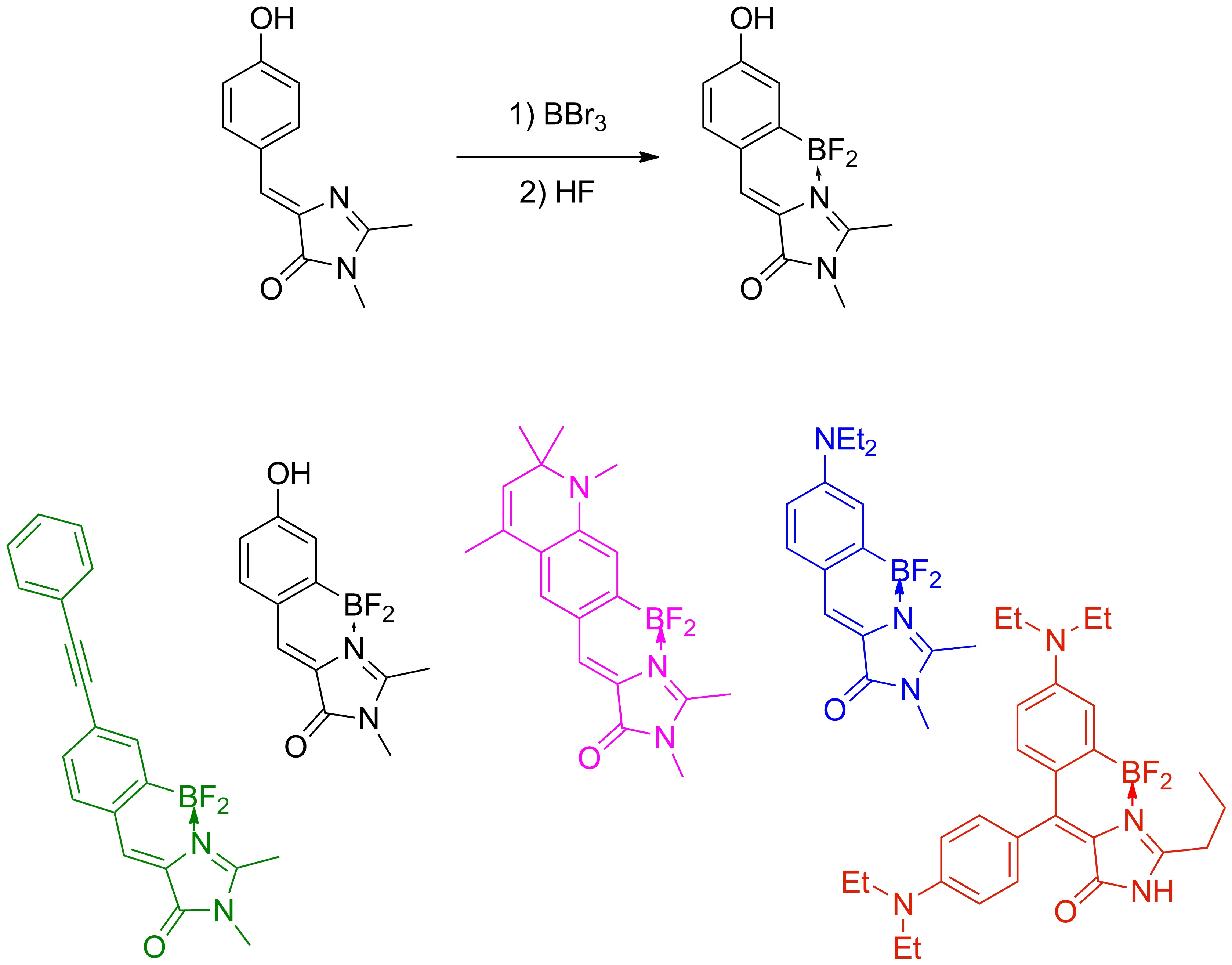
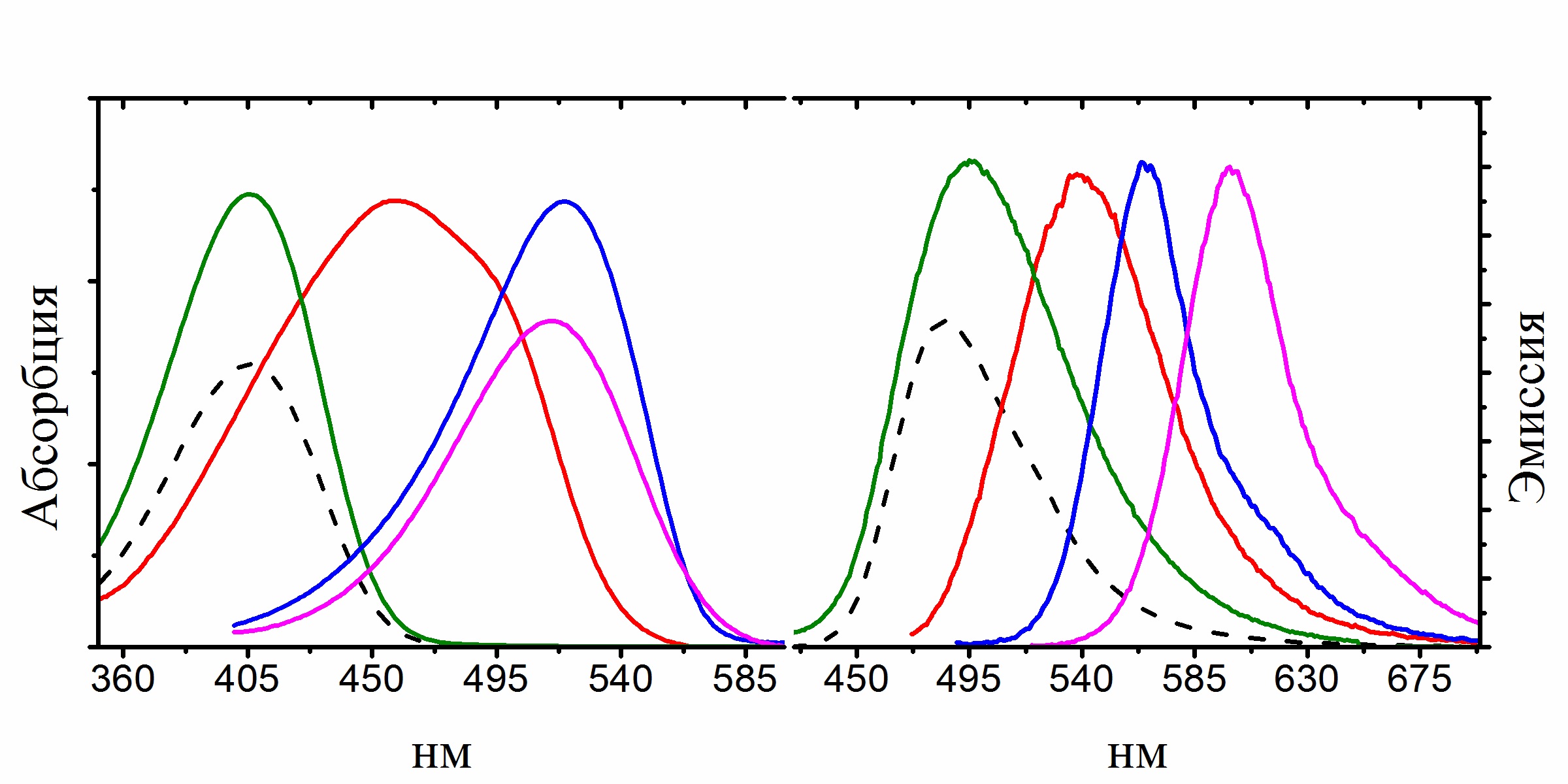
Existing fluorescent dyes used for staining living systems have a number of significant disadvantages and are unsuitable for solving a certain range of problems (for example, there are practically no compounds with Stokes shifts of more than 100 nm among them). At the same time, the chromophores of fluorescent proteins do not have many of the disadvantages inherent in the existing dyes, and therefore they are an excellent basis for creating new dyes.
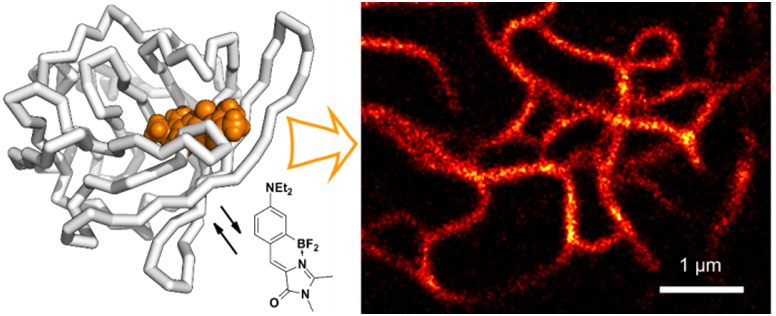
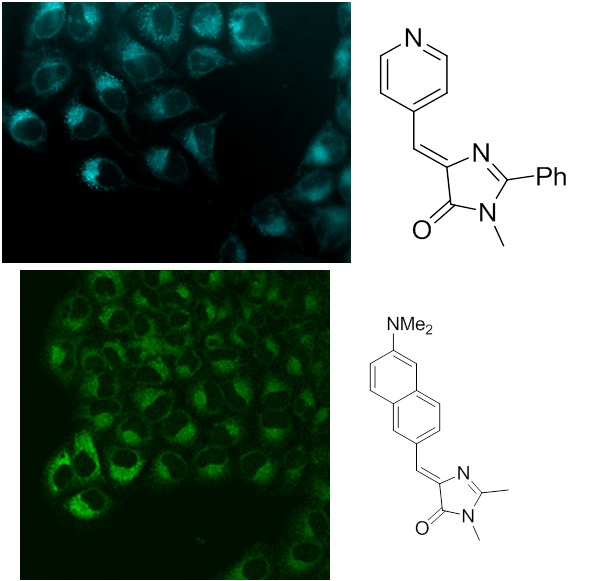
Development of new fluorogenic dyes, including ones based on the chromophores of fluorescent proteins
One of the new and modern methods of fluorescent labeling of biological cells is the use of so-called fluorogenic dyes – substances, which do not have a pronounced fluorescence in a free form and acquire it only when bound to the target object.
One of the promising candidates for the role of such substances are the chromophores of fluorescent proteins and their derivatives.
In this regard, in our group, the creation and study of various fluorogenic compounds is actively conducted.
Development of new approaches to the synetze of heterocyclic compounds
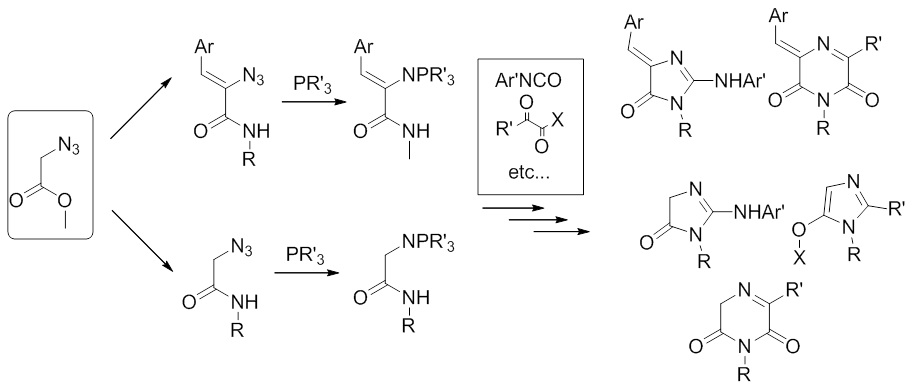
Our team has long been studying the chemistry of chromophores of fluorescent proteins based on the molecule - 4-benzylidene-imidazole-5-ones. During this work we have created several new approaches to the synthesis of these compounds, and in parallel many unexpected transformations associated with the use of esters of nitroacetic and azidoacetic acids were discovered.
In particular, the method of synthesis of 5-hydroxy-1,2-oxazine-6-ones discovered by us allows us to take a new look at one of the methods for the synthesis of isoxazole-3,5-dicarboxylic acid derivatives, the Dornow reaction.
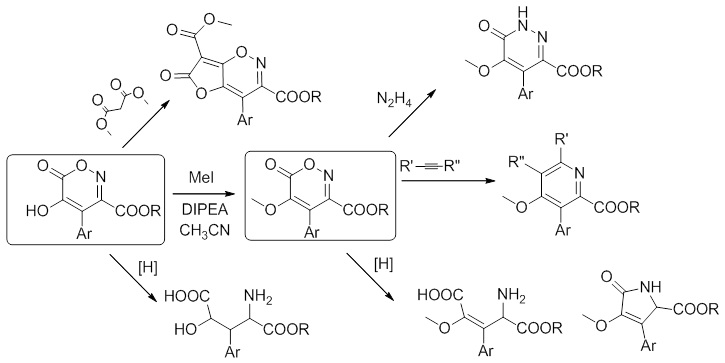
Similarly, the transformations of the derivatives of azidoacetic acid and their phosphazenes observed by us are also not reflected in the scientific literature, which suggests the possibility of creating new ways of synthesizing heterocyclic systems and from these reagents.
| Fullname | Position | Contacts |
|---|---|---|
| Mikhail Baranov, D.Sc | Head of lab., l. r. f. | baranovmikes@gmail.com |
| Mikhail Baranov, D.Sc | Head of lab., l. r. f. | baranovmikes@gmail.com |
| Yulia Bogdanova, Ph.D. | r. f. | |
| Aidar Gil'vanov | j. r. f. | |
| Ivan Myasnyanko, Ph.D. | j. r. f. | |
| Alexander Smirnov, Ph.D. | j. r. f. | |
| Alekseeva I.A. | t. q. - lab. as. | |
| Dmitrii Ivanov | t. q. - lab. as. | |
| Krasnova S.A. | t. q. - lab. as. | |
| Lyukmanova G.R. | t. q. - lab. as. | |
| Marina Molchanova | t. q. - lab. as. | |
| Fradkov A.F., Ph.D. | eng. | |
| Mustafin D.A. | eng. | |
| Oprishko V.E. | eng. | |
| Daniil Rudik | eng. | |
Previously worked here | ||
| Nadezhda Baleeva, Ph.D. | ||
| Alexey Nizovtsev, Ph.D. | ||
| Anatoliy Sokolov | ||
| Elvira Zajceva | ||
| Zaytseva S. | ||
| Tyushina I.V. | ||
| Yunisova G.R. | ||
| Nikolaev S.E. | ||
| Elena Sokolinskaya, Ph.D. | elena.sokolinskaya@gmail.com | |
| Ekaterina Zhigileva | ||
 Loading...
Loading...Scientific projects
 Loading...
Loading...Mikhail Baranov
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
ORCID: 0000-0002-9339-7603, Scopus: 55170275100, ResearcherID: L-5014-2016
 Loading...
Loading...
