Лаборатория лиганд-рецепторных взаимодействий
Лаборатория организована в 2009 г. и возглавляется доктором химических наук И. Е. Кашеверовым.
Основными задачами лаборатории лиганд-рецепторных взаимодействий являются структурно-функциональные исследования некоторых Cys-петельных рецепторов (никотиновые, глициновый) с помощью пептидных лигандов природного происхождения и их искусственных аналогов, а также создание новых биологически активных пептидных соединений на основе известных холинергических лигандов.
В лаборатории проводятся работы по компьютерному дизайну новых соединений на базе структур известных холинергических лигандов (конотоксины разных классов) и моделей их комплексов с никотиновыми рецепторами или их гомологами (ацетилхолин-связывающие белки), химическому синтезу различных пептидных соединений и анализу их биологической активности с применением различных методов исследования (радиолигандный анализ, электрофизиология, плазмонный резонанс).
В лаборатории было синтезировано (в том числе и на основе дизайна с применением методов компьютерного моделирования) более 4-х десятков различных конотоксинов и их аналогов, часть из которых в дальнейшем модифицировалась радиоактивными и/или фотоактивируемыми метками. С помощью некоторых из них были структурно охарактеризованы лиганд-связывающие участки природного никотинового рецептора. С использованием другого аналога, оказавшегося более активным соединением, чем природный конотоксин, впервые была установлена кристаллическая структура ацетилхолин-связывающего белка в комплексе с пептидным антагонистом и выявлены ее принципиальные отличия от структуры комплексов с агонистами. Полученные данные стали основой для создания компьютерных моделей различных типов никотиновых холинорецепторов.
Часть представленной работы нашла отражение в недавно опубликованных обзорах: Tsetlin V., Utkin Y., Kasheverov I. (2009). Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem Pharmacol. 78(7), 720—731 иKasheverov I.E., Utkin Y.N., Tsetlin V.I. (2009). Naturally occurring and synthetic peptides acting on nicotinic acetylcholine receptors. Curr Pharm Des. 15(21), 2430—2452.
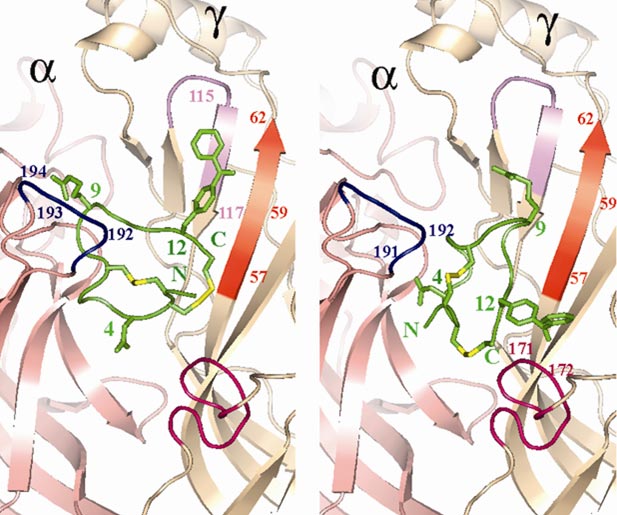
Модель комплекса аналога α-конотоксина GI с природным холинорецептором Torpedo: предложены две возможные ориентации пептида в кармане связывания между a- и γ-субъединицами на основании данных метода фотоаффинной модификации. Kasheverov IE, et al. FEBS J. 273(7), 1373—1388 (2006).
| ФИО | Должность | Контакты |
|---|---|---|
| Кашеверов Игорь Евгеньевич, д.х.н. | зав. лаб., г.н.с. | |
| Кашеверов Игорь Евгеньевич, д.х.н. | зав. лаб., г.н.с. | |
| Крюкова Елена Викторовна, к.х.н. | с.н.с. | |
| Кудрявцев Денис Сергеевич, к.б.н. | с.н.с. | kudryavtsev@ibch.ru |
| Можаева Вера Александровна | м.н.с. | Veramozhaev@yandex.ru |
| Архангельская П.С. | тех.-лаб. | |
| Гондаренко Е.А. | м.н.с. | |
| Беляева А.Ю. | тех.-лаб. | |
| Рудова А.Л. | тех.-лаб. | |
| Стукалова Анна Юрьевна | тех.-лаб. | |
Ранее здесь работали | ||
| Жмак Максим Нургаянович, к.х.н. | ||
| Райтман О.А., к.х.н. | ||
| Рябинин В.В. | ||
| Иванов Игорь Андреевич | ||
| Лебедев Д.С., к.б.н. | ||
| Макарова Я.В. | ||
| Копылова Н.В. | ||
| Николаев Г.М. | ||
| Сон Лина Викторовна | ||
| Спирова Е.Н., к.б.н. | ||
| Воронцова О.В. | ||
| Исаева А.С. | ||
| Скрипка М.И. | ||
| Кузьмин Д.А. | ||
| Хрущев А.Ю. | ||
| Белан Д.В. | ||
| Майоров В.А. | ||
| Михайленко А.Д. | ||
| Рузаева Е.К. | ||
| Сенько Д.А. | ||
| Холошенко Инна Владимировна | ||
| Швецова М.А. | ||
| Галеев А.Р. | ||
| Гринкина С.Д. | ||
| Егоров А.В. | ||
| Карамышева Н.Д. | ||
| Нигматулина Н.Б. | ||
| Прусс И.В. | ||
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Кашеверов Игорь Евгеньевич
Москва, ул. Миклухо-Маклая, 16/10 — На карте
ORCID: 0000-0002-7373-6524, ResearcherID: F-6024-2014, Scopus: 6701702701
 Загрузка...
Загрузка...
