Отдел молекулярной нейроиммунной сигнализации

|
Руководитель: Цетлин Виктор Ионович В составе подразделения: |
Отдел организован в 2009 г. и возглавляется чл.-корр. РАН профессором В. И. Цетлиным. Основными задачами отдела являются исследования молекулярных механизмов, лежащих в основе нейросигнализации, а также развитие и совершенствование методов исследования процессов нейросигнализации. В основе работы отдела лежит тесное взаимодействие Лаборатории лиганд-рецепторных взаимодействий и Лаборатории молекулярной токсинологии, владеющих взаимодополняемыми современными методами структурной нейробиологии.
Исследования отдела в основном сконцентрированы на механизме взаимодействия конкурентных антагонистов α-конотоксинов и α-нейротоксинов с никотиновым холинорецептором. С использованием различных подходов (в частности широко применялись фотоактивируемые производные токсинов) установлены основные структурные особенности участков связывания агонистов/конкурентных антагонистов холинорецептора мышечного типа.
Конотоксины и нейротоксины змей по-прежнему используются в качестве инструментов исследования, однако набор изучаемых рецепторов значительно расширился и включает, в частности, ацетилхолин-связывающие белки и некоторые Cys-петельные рецепторы. Работы проводятся в тесном сотрудничестве с зарубежными коллегами (профессора Smit (Амстердам), Betz (Франкфурт-на-Майне) и др.). В частности, в сотрудничестве с коллегами из Амстердама была впервые установлена кристаллическая структура ацетилхолин-связывающего белка в комплексе конотоксином (Nature Str.&Mol.Biol. 12, 582—588 (2005)).
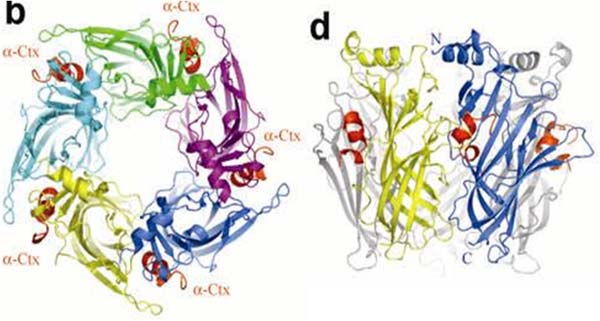
Кристаллическая структура α-конотоксина PnIA[A10L, D14K] в комплексе с ацетилхолин-связывающим белком A.californica. Celie P.H.N., Kasheverov I.E., et al., 2005.
 Загрузка...
Загрузка...Цетлин Виктор Ионович
Москва, ул. Миклухо-Маклая, 16/10 — На карте
ORCID: 0000-0002-7980-6191, Scopus: 7006181582
 Загрузка...
Загрузка...
