Лаборатория молекулярной иммунологии
|
Руководитель: Деев Сергей Михайлович |

Дружный коллектив Лаборатории молекулярной иммунологии на Дне Здоровья.
Видео - собирательный образ выездных семинаров лаборатории на Москве-реке Дни здоровья 2008-2011
Интервью с заведующим лабораторией Деевым Сергеем Михайловичем, опубликованное в журнале "Наука из первых рук" Самонаводящееся лекарство.pdf (2017).
Вручение премии Президента в области науки и инноваций для молодых учёных за 2017 год старшему научному сотруднику лаборатории - Никитину Максиму Петровичу Видеозапись церемонии
В лаборатории выполняется проект КОМФИ 17-00-00121-Н "Новые подходы к адресной терапии злокачественных новообразований с использованием инновационного направляющего модуля неиммуноглобулиновой природы", 2017-2020 гг. Проект КОМФИ_2017.pdf
В лаборатории выполняется проект РФФИ 17-34-80105 мол_эв_а "Новые подходы к поверхностной модификации наноструктур с целью создания эффективных агентов для тераностики", 2017-2018. РФФИ 17-34-80105.pdf
В лаборатории закончен проект РНФ №14-24-00106 "Комплексный подход к биоинженерии мультифункциональных соединений направленного действия для диагностики и терапии рака", 2017-2018 гг. Основные результаты 2018 года: РНФ №14-24-00106п_2018.pdf Основные результаты 2017-2018 гг.: РНФ №14-24-00106п_2017-2018.pdf
В лаб. молекулярной иммунологии ИБХ РАН завершен Научный проект РФФИ_КОМФИ 17-00-00121-Н "Новые подходы к адресной терапии злокачественных новообразований с использованием инновационного направляющего модуля неиммуноглобулиновой природы", который является интегральной составляющей Комплексного проекта КОМФИ 17-00-00122-К "Разработка комплекса новых технологий для тераностики онкологических заболеваний: мультифункциональные биосовместимые агенты, сверхчувствительные системы люминесцентной диагностики и биологические модели для прижизненного тестирования", выполненного в 2017-2020 гг. совместно с коллективами №2 (ФНИЦ «Кристаллография и фотоника») и № 3 (ННГУ им. Н.И. Лобачевского) и направленного на создание комплекса новых технологий для высокоселективной оптической диагностики и эффективной терапии онкологических заболеваний. Основные результаты, полученные в ходе выполнения Комплексного проекта см. здесь: Стенд_Комплексный.pdf
Основные результаты, полученные в ходе выполнения научного проекта КОМФИ 17-00-00121-Н "Новые подходы к адресной терапии злокачественных новообразований с использованием инновационного направляющего модуля неиммуноглобулиновой природы см. здесь: Результаты_РФФИ_КОМФИ_17-00-00121.pdf.
2017 год.
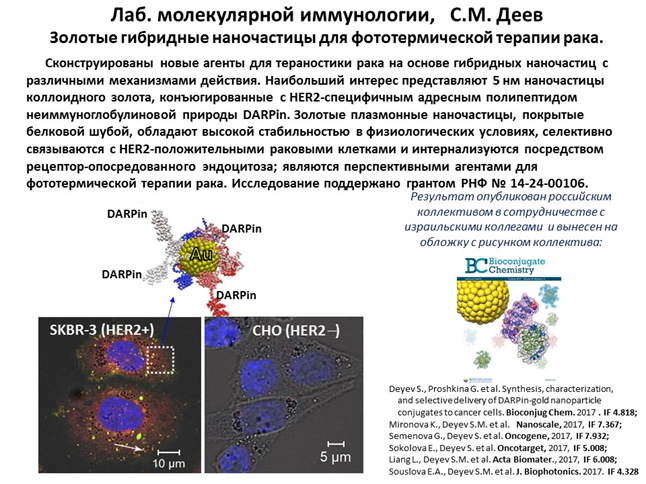
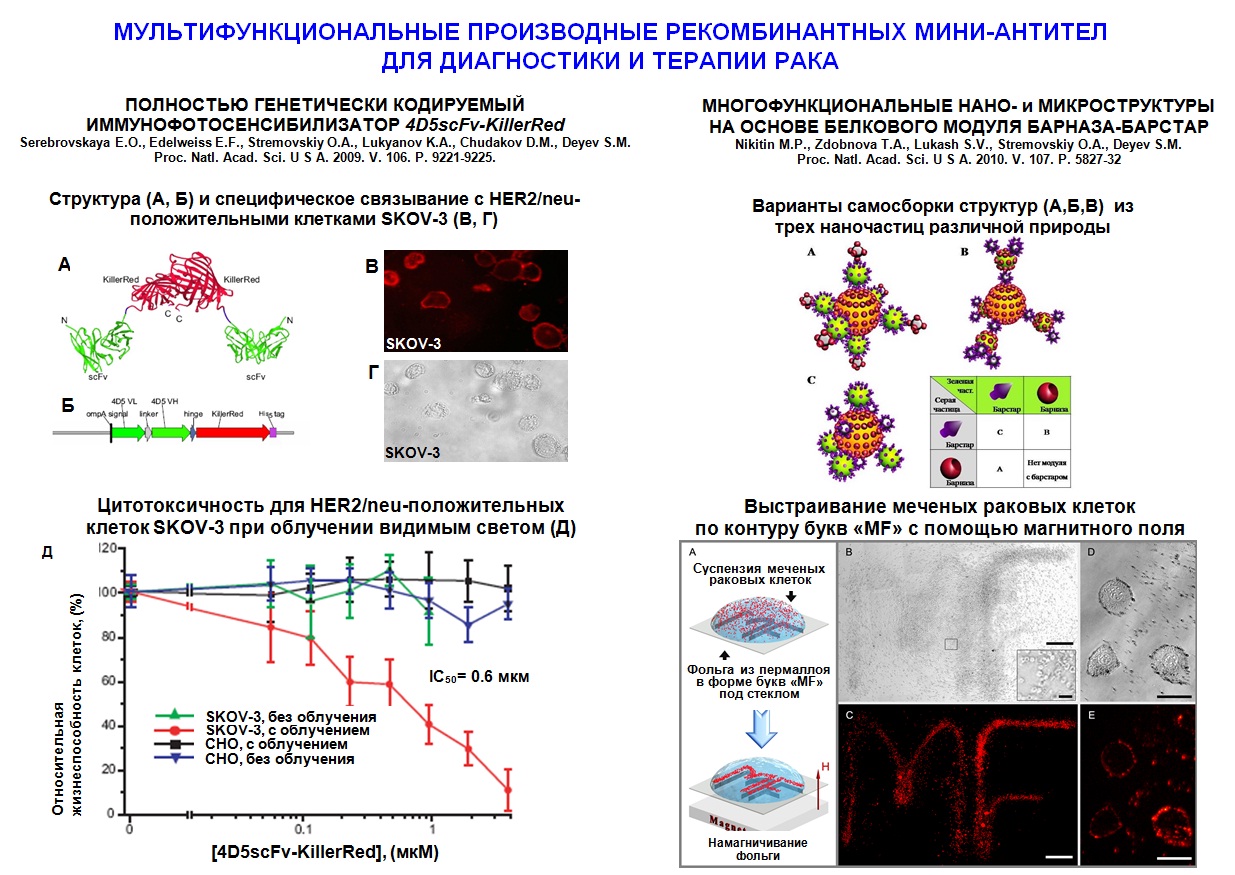
2010 год. На основе разработанных принципов сконструированы и охарактеризованы два новых высокоэффективных иммунотоксина направленного действия для адресного поражения патогенных клеток. Показано, что бифункциональное производное рекомбинантных анти-HER2/neu-мини-антител - полностью генетически кодируемый иммунофотосенсибилизатор на основе фототоксического белка KillerRed – адресно поражает при облучении опухолевые клетки, гиперэкспрессирующие онкомаркер HER2/neu, причем его действие усиливается при сочетанном применении традиционного химиотерапевтического агента - цисплатина. Второй иммунотоксин, нацеленный на модельные В-клетки с помощью специфического эпитопа, вызывает апоптоз клеток-мишеней за счет действующего агента - рибонуклеазы барназы и характеризуется оптимальным соотношением токсичности и селективности.
На основе разработанных принципов созданы и охарактеризованы гибридные биосовместимые флуоресцентные нанокомплексы разной природы для визуализации опухолевых клеток человека in vitro и in vivo. Впервые на универсальной платформе белкового модуля барназа-барстар созданы конъюгаты люминесцентных наноалмазов с флуоресцентным белком EGFP и с золотыми наночастицами. Полученные наночастицы устойчивы в виде водных суспензий в течение длительного времени, способные неспецифически метить эукариотические клетки, обладают широким спектром излучения и фотостабильностью. Путем включения в полимерные частицы получены стабильные водные суспензии флуоресцентных нанокристаллов (квантовых точек) и их конъюгатов с противораковыми мини-антителами. Полученные соединения специфически метят раковые клетки и характеризуются высоким квантовым выходом и фотостабильностью. В работе получен набор флуоресцентных полимерных частиц, в том числе, содержащих КТ, отвечающих требованиям биоанализа, и проведена оценка возможности использования полученных частиц для визуализации биомолекул на примере реакции латексной агглютинации и маркирования клеточных рецепторов. Высокая эффективность флуоресценции, уникальная фотостабильность синтезированных полимерных частиц, разнообразие цветов флуоресценции при использовании одного источника возбуждения открывают широкие возможности для применения полимерных частиц в медицине и биологии.
Предложен уникальный метод самосборки в единые суперструктуры заранее программируемого состава различных компонентов, например магнитных частиц, квантовых точек и антител, независимо от свойств их поверхности. Создаваемые с помощью разработанного метода частицы могут использоваться в новейшей области медицины - терагностике, в которой один препарат используется как для диагностики, и для терапии заболеваний.
Обзор в журнале "Биохимия": "ERBB онкогены - мишени моноклональных антител".
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Деев Сергей Михайлович
Москва, ул. Миклухо-Маклая, 16/10 — На карте
ResearcherID: F-8191-2014, Scopus: 6603799895
 Загрузка...
Загрузка...
