Press-room / news / Science news /
Plasma protein corona of liposomes loaded with a phospholipid–allocolchicinoid conjugate enhances their anti-inflammatory potential
The staff of the Laboratory of Lipid Chemistry IBCh RAS, together with colleagues from Nizhny Novgorod State University and the Human Proteome Center of the Institute of Biomedical Chemistry, studied the effects of protein coronas formed in human blood plasma ex vivo on different liposomes carrying a colchicine analog. Today colchicine is considered as a possible treatment for cardiovascular complications. On a model of monocytes from human peripheral blood, the protein coronas have been shown to enhance the anti-inflammatory potential of liposomes. A particularly sharp effect was observed for liposomes bearing specific proteins, including minor ones in normal plasma.
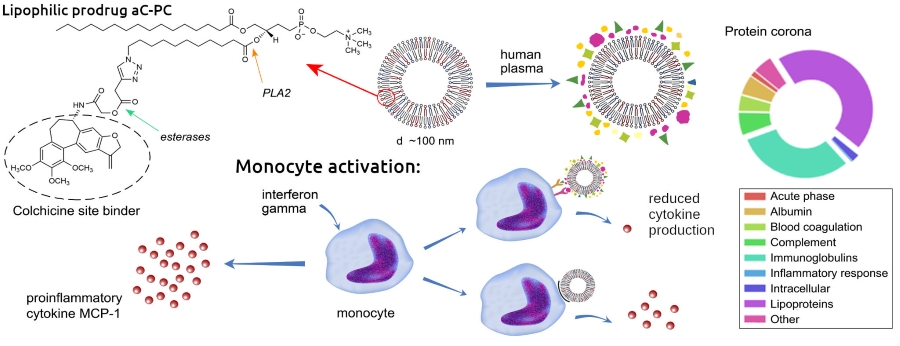
Today colchicine is considered as a possible new treatment for cardiovascular complications, including for blocking the development of inflammation after a myocardial infarction. As shown by pharmacokinetic/pharmacodynamic studies, the biological effects of colchicine are mainly related to intra-leukocyte concentrations, and not to plasma concentrations. Nanoscale liposome carriers of colchicine can provide its delivery to cells involved in inflammation, including monocytes and endothelial cells. Colchicine is a neutral lipophilic molecule, which localizes mainly in the lipidic environment, however, it is prone to being expelled to the aqueous phase with formation of stacked aggregates. Previously, colleagues from Nizhny Novgorod State University synthesized a phospholipid conjugate of a colchicine analog (aC-PC), which, after hydrolysis by phospholipase A2 and esterases, releases an allolchicinoid capable of blocking the colchicine site of tubulin. In collaboration, we formulated liposomes based on egg phosphatidylcholine and palmitoyl oleoylphosphatidylglycerol (POPG) with a different content of aC-PC conjugate in the bilayer. The entire series of liposomes showed low cytotoxicity against human cultural endotheliocytes and monocytes. And what effects can be expected with the systemic administration of these liposomes? As we know, when liposomes enter the bloodstream, like other nanoparticles, they are instantly covered with a complex layer of proteins and lipoproteins that mediate their interactions with mononuclear cells and other cells, including target ones. The structure of the protein corona depends on the physico-chemical properties of the liposome surface, which is unique for various lipid formulations. Together with the Human Proteome Center of the Institute of Biomedical Chemistry we studied the protein coronas of liposomes with different contents of aC-PC and POPG. Liposome-protein complexes were isolated after incubation in the pooled blood plasma of 44 healthy donors. According to proteomic analysis, the main proteins in the coronas of different liposomes were apolipoproteins and immunoglobulins, but their ratios, as well as the content of proteins of other classes, differed significantly. The top-10 proteins in the corona of liposomes 25C, with the maximum content of aC-PC conjugate (25 mol. %), included proteins not represented in other formulations: C4B-binding protein, inhibitor of the classical and lectin pathways of the complement system activation, apolipoprotein D and galectin-3-binding protein (minor in blood plasma normally). To evaluate the anti-inflammatory potential of liposomes, we modeled the inflammatory process on monocytes from the peripheral blood of healthy donors. The cells were stimulated with interferon-gamma, then incubated in a donor plasma medium and the production of chemokines MCP-1 and interleukin 8 was monitored to confirm the status of inflammation. After that, cells were incubated with liposome-protein complexes obtained in donor plasma and with liposomes without coronas, and the production of chemokines was checked. All liposomes, even the control ones (without aC-PC), with and without coronas, inhibited IL-8 production by about 2 times. In the case of MCP-1, a significant effect of coronas was found: the release of chemokine decreased 1.5 times under the action of liposomes with protein coronas, compared with naked liposomes, and liposomes 25C with a corona completely suppressed the production of MCP-1. Presumably, the strong anti-inflammatory effect of the corona of these liposomes is mediated by apoD and galectin-3-binding protein, which are involved in the inflammation-associated signaling. Liposomes 25C are considered the most promising for further preclinical studies on an adequate model of cardiovascular complications in vivo.
The results are published in Colloids and Surfaces B: Biointerfaces.
may 12, 2025


