Laboratory of Chemistry of Metabolic Pathways
The Laboratory was established in 2017 on the basis of the Total Synthesis Group which was created in the Laboratory of Molecular technologies at IBCH RAS, headed by Sergey Lukyanov. The mission of the group is to apply organic synthesis to solving actual problems in biochemistry, molecular biology and medicinal chemistry.
This includes:
- structural design and synthesis of model compounds for studying biochemical processes
- total synthesis of natural products
- design, synthesis and testing of drug candidates
- offering our expertise in organic synthesis to biological and biomedical researchers in collaborative projects
 |  |
 | |
LATEST NEWS

Our Accounts of Chemical Research (ACS) cover with review of the latest developments in bioluminescent systems research of our group
CURRENT
- Studies of fungal bioluminescence

Photograph by Professor Cassius Stevani (University of San Paulo, Brazil)
The Guardian about our work
- Studying bioluminescence of marine worms Chaetopterus variopedatus
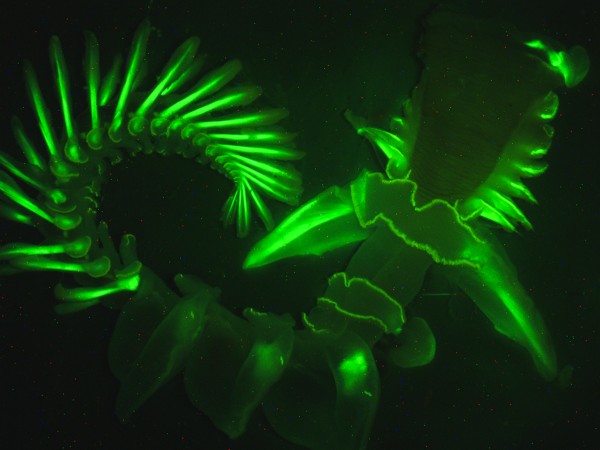
Photograph courtesy Dimitri Deheyn, Scripps Institution of Oceanography at UC San Diego
- Studying Studying bioluminescence mechanism of Siberian earthworms Fridericia heliota
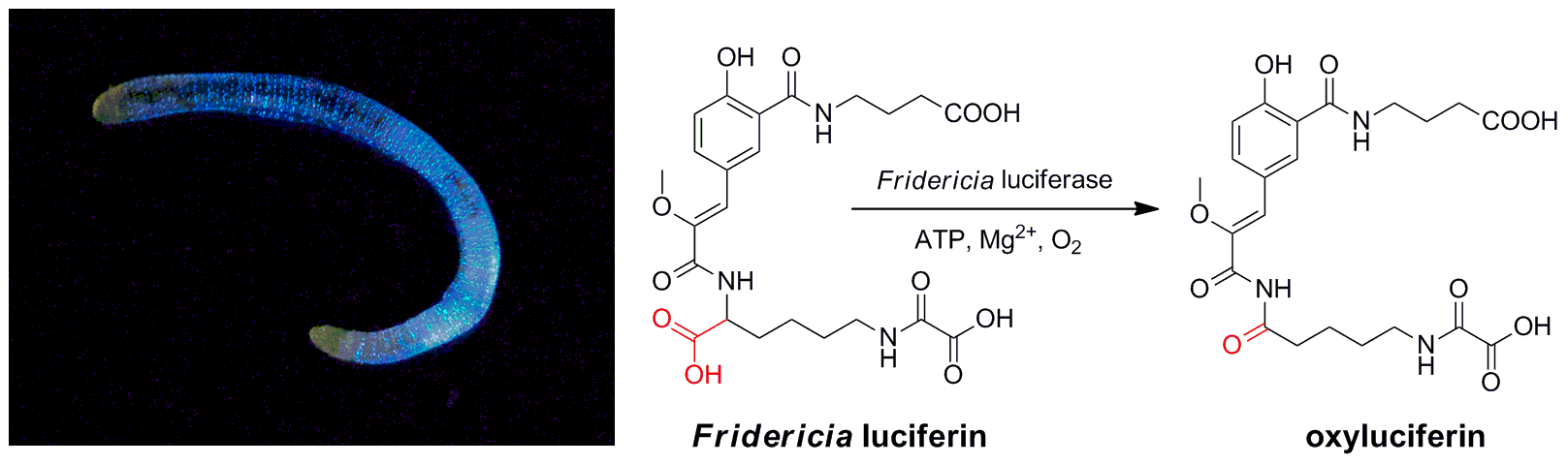
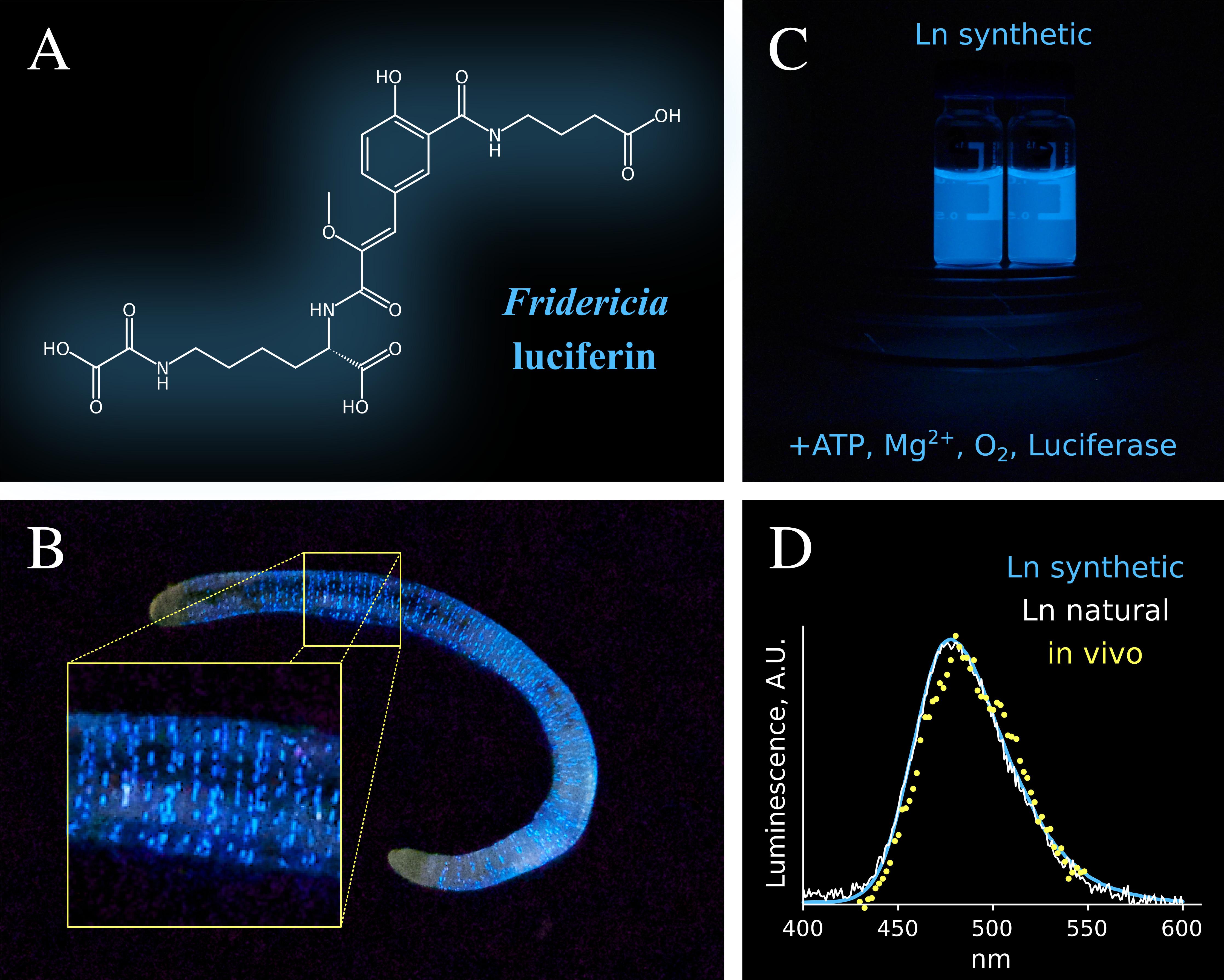
(А) Structure of Fridericia luciferin. (B) Bioluminescence of Fridericia heliota. The photograph is courtesy of Alexander Semenov (the White Sea Biological Station, Biology Department of Lomonosov Moscow State University). (C) Luminescence of synthetic Fridericia luciferin . (D) Comparison of in vivo bioluminescence spectra of worms, with in vitro bioluminescence spectra of natural and synthetic samples of luciferin.
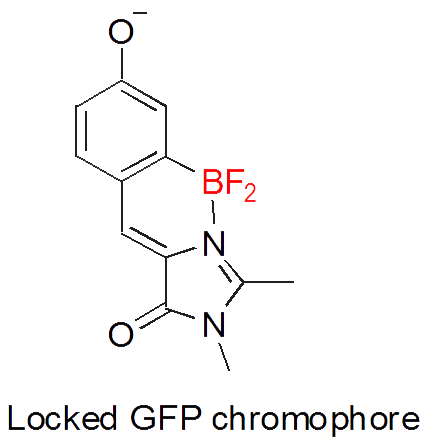
- Development of novel class of fluorescent dyes based on GFP chromophore
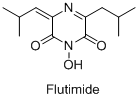
- Development of anti-influenza drug candidates based on structural analogs of Flutimide
- Enantioselective total synthesis of fungal terpenoid panal from bioluminescent fungus Panellus stipticus

Panellus stipticus
- Studying luminescence mechanism of luminous fungi

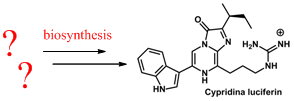
- Studying biosynthetic origin of marine luciferins: coelenterazine and Cypridina luciferin
Cypridina hilgendorfii
- Developing fluorogenic sensors to important protein targets

FULFILLED
2009-2011
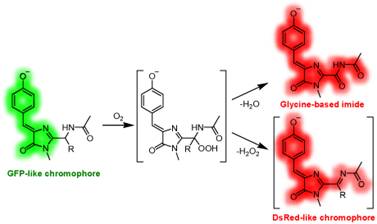
Biosynthetic mechanism of chemically unstable acylimine chromophore of red fluorescent proteins was investigated. This required development of new synthetic strategy to 2-acylaminoimidazolones - biosynthetic precursors of 2-acyliminoimidazolones. We have shown spontaneous oxidation of precursors with air oxygen by unusual mechanism.

Discosoma
2002-2009
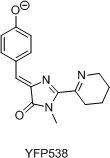
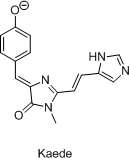
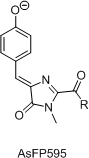
Total synthesis of chromophores of GFP-like fluorescent proteins was used as a tool for independent structure determination of these chromophores. Also, this allowed to study structure-spectral properties relationship within this range of imidazole derivatives and to develop novel synthetic approaches to 2-functionalized arylideneimidazolones. Chromophores of AsFP595, Kaede and YFP538 were synthesized.

Zoanthus

Trachyphyllia

Anemonia
2002-2004
A blue proteinaceous pigment from Rhizostoma pulmo (jellyfish from the Black Sea) was isolated, sequenced and studied by biochemical methods. An attempt to determine the nature of the blue coloration (chromophore) was unsuccessful.


Black Sea jellyfish Rhizostoma pulmo possessing an unidentified blue pigment
 Loading...
Loading...Scientific projects
 Loading...
Loading...Ilia Yampolsky
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
ORCID: 0000-0003-2558-2476, Scopus: 7801466424
 Loading...
Loading...
