Laboratory of Biopharmaceutical Technologies
The main focus of the group of genetically engineered biopharmaceutical technologies are the development of fundamental and applied aspects of biotechnology and the application of own technologies in the production of biopharmaceutical drugs. The group carries out a full cycle of works in genetic engineering and protein chemistry ‒ from choosing the gene cloning strategy and its chemical enzymatic synthesis to developing a method for isolation, purification, complete physical and chemical identification and research of biological activity, expression scaling and fermentation stage, and also the creation of laboratory and pilot manufacturing technology protocol, which are implemented in conjunction with the Experimental Biotechnological plant (EBP). Special attention is paid to the development of biotechnologies for the production of biologically active pharmaceutical substances (APS) and the creation of finished pharmaceutical product (FPP) based on recombinant proteins and peptides, fullfilled by the Experimental Biotechnological plant (EBP) of IBCh RAS.
The group, which is part of the laboratory of biotechnology, successfully participates in government programs: Pharma 2020, the Federal Space Program of Russia, grants from the Russian Science Foundation and the Russian Foundation for Basic Research. The team members are the winners of the competition of young scientists at the Moscow International Conference "Biotechnology and Medicine", "UMNIK".
The team members have extensive experience in creating and further verifying a wide range of recombinant proteins produced using protein splicing technologies. The teamwork of the group and the Experimental Biotechnological plant of the IBCh RAS allowed in the shortest possible time to organize the manufacturing of pilot batches of potential drugs within the framework of state contracts of the Ministry of Industry and Trade and the Ministry of Education and Science for preclinical studies.
Much attention in the group is paid to work with schoolchildren, students and graduate students of specialized educational institutions. Zayats Evgeny Andreevich and Tereshin Mikhail Nikolaevich, constantly working in the group, completed a master's degree in the Institute of Fine Chemical Technologies of Moscow Technological University (MTU). During the student years, they actively participated in the several intellectual biological tournaments of the All-Russian and international level as part of the BioTech team of MIT: IV Open All-Russian student team competition "Bioturnir 2017" in c. Pushchino (II place in the team competition), International Biological Universiade MSU 2017 (2sd level degree of command diploma), II Student Biological Tournament MSU 2018 (3rd level degree of command diploma).
On October 27, 2016, employees of the Institute of Bioorganic Chemistry of the Russian Academy of Sciences conducted an excursion for the participants of the vocational guidance project of the Bauman Library ON. Nekrasov "Tomorrow".
One of the main achievements of the group of genetically engineered biopharmaceutical technologies together with the Experimental Biotechnological plant (EBP) of of IBCh RAS is the development and implementation of domestic technology the production of active pharmaceutical substance (APS) of human genetically engineered glucagon (headed by Acad. AI Miroshnikov). The project was implemented in 2012-2015 with the support of the Ministry of Industry and Trade of the Russian Federation. Obtaining of APS was the beginning of developmen of the finished pharmaceutical form of glucagon - “GLUCORAN” (glucagon recombinant, human genetic engineering, lyophilisate for preparing injection solution of 1 IU / mg). Completion of this project made it possible to prepare a drug registration dossier for production on the basis of EBP of IBCh RAS and pharmaceutical forms of human genetic glucagon.
Under the leadership of Roman S. Esipov, 4 candidates and more than 25 bachelor`s and master's degrees have been defended.
PhD thesis Makarov D.A.

Partners:
- R-Pharm JSC
- Prof. I.P. Kuranova - Head. Laboratory of Protein Crystallography; Institute of Crystallography. Shubnikova RAS
- Ph.D. S.P. Domogatsky - Head. Engineering Immunology Group, Russian Cardiological Research and Production Complex, Ministry of Health of the Russian Federation Institute of Experimental Cardiology
- Prof. V.S. Akopyan - Head Department of Ophthalmology Faculty of Fundamental Medicine, MSU
- Prof. М.А.Vladimirsky - Head of the Laboratory of Immunological Research and Molecular Diagnosis of Tuberculosis Research Institute of Physiopulmonology of the First Sechenov Moscow State University
- Ph.D. M. Filippenko - Head of the Laboratory of Pharmacogenomics Research Institute of Chemical Biology and Fundamental Medicine
- D.Sc. Yu.S. Skoblov - Head of the Laboratory of Isotopic Methods of Analysis Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences
- Prof. S.N.Mikhailov - Head of the Laboratory of Nucleoside Engelhardt Institute of Molecular Biology (EIMB), RAS
- Scientific Center "Signal" (FGUP NTS Signal)
- Research Institute for Applied Acoustics (FSUE NIIP)

Biopharmaceutical product:
- A biotechnological approach has been developed to produce a number of active pharmaceutical substances of recombinant polypeptides based on fusion proteins containing thioredoxin A, the target polypeptide, and the cleavage site of the TEV proteinase.
- An intein-mediated biotechnological approach has been developed to obtain a number of active pharmaceutical substances of recombinant polypeptides based on a fusion protein containing the protein splicing elements (inteins) and not requiring the use of specific proteases.
- A biotechnological technology for producing recombinant thymosin α1 and human thymosin β4 without the stage of chemical acetylation has been developed
- The pilot manufacturing technology protocols for the preparation of active pharmaceutical substances of the thymosin α1 (Redaxin), thymosin β4 (Thymoran), glucagon (Glucoran), a modified fragments of the tumstatin (Tumatistin) and pigment epithelium-derived factor (Pigostin), analogs of hirudin (Lepirudin and Desirudin), endostatin, sialylation oxintomodulin (Oxilong), epidermal growth factor, oxytocin, analgesic ARNS-3 (Peptalgin), analgesic RT-1 (Punalgin) were developed using the developed technological approaches.
- On the basis of the APS the following drugs "Glucoran", "Oksilong", "Pigostin", "Peptalgin", "Timoran", "Punalgin" were created.
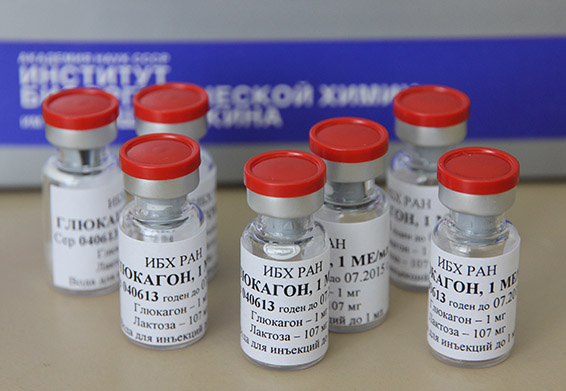
An active pharmaceutical substance obtained for preclinical trials.
- Technological control methods and analysis of the final product, including testing of biological activity, have been developed for all the created technologies.
- Technological documentation has been developed (pilot manufacturing technology protocols).
- The high specific biological activity of the “Timoran”, “Glucoran”, “Oksilong”, “Pigostin”, “Tumstatin”, “Lepirudin”, “Desirudin”, “Peptalgin” and “Punalgin” has been proven on cellular and animal models.
- Laboratory procedures have been developed for obtaining a series of cytokines, including their pegylated form: interleukin 2, interleukin 3, interleukin 4, interleukin 15, interleukin 18, interferon alfa-2a, interferon alfa-2b.
Tuberculosis field:
- The strain-producers of target enzymes from Mycobacterium tuberculosis, vital for the activity of mycobacteria, and methods for purification of these enzymes are developed:
- acid phosphatase
- phosphopanthetein adenylyltransferase
- Ser / Thr-specific protein kinase
- imidazole-glycerol-phosphate dehydratase
- Aspartate AspC Aminotransferase
- ketol-acid reductoisomerase
- By the method of counter diffusion in capillaries, crystals of mycobacterial phosphopanthetein-adenylyltransferase complex with substrates - ATP, coenzyme A and dephosphocoenzyme A were obtained.
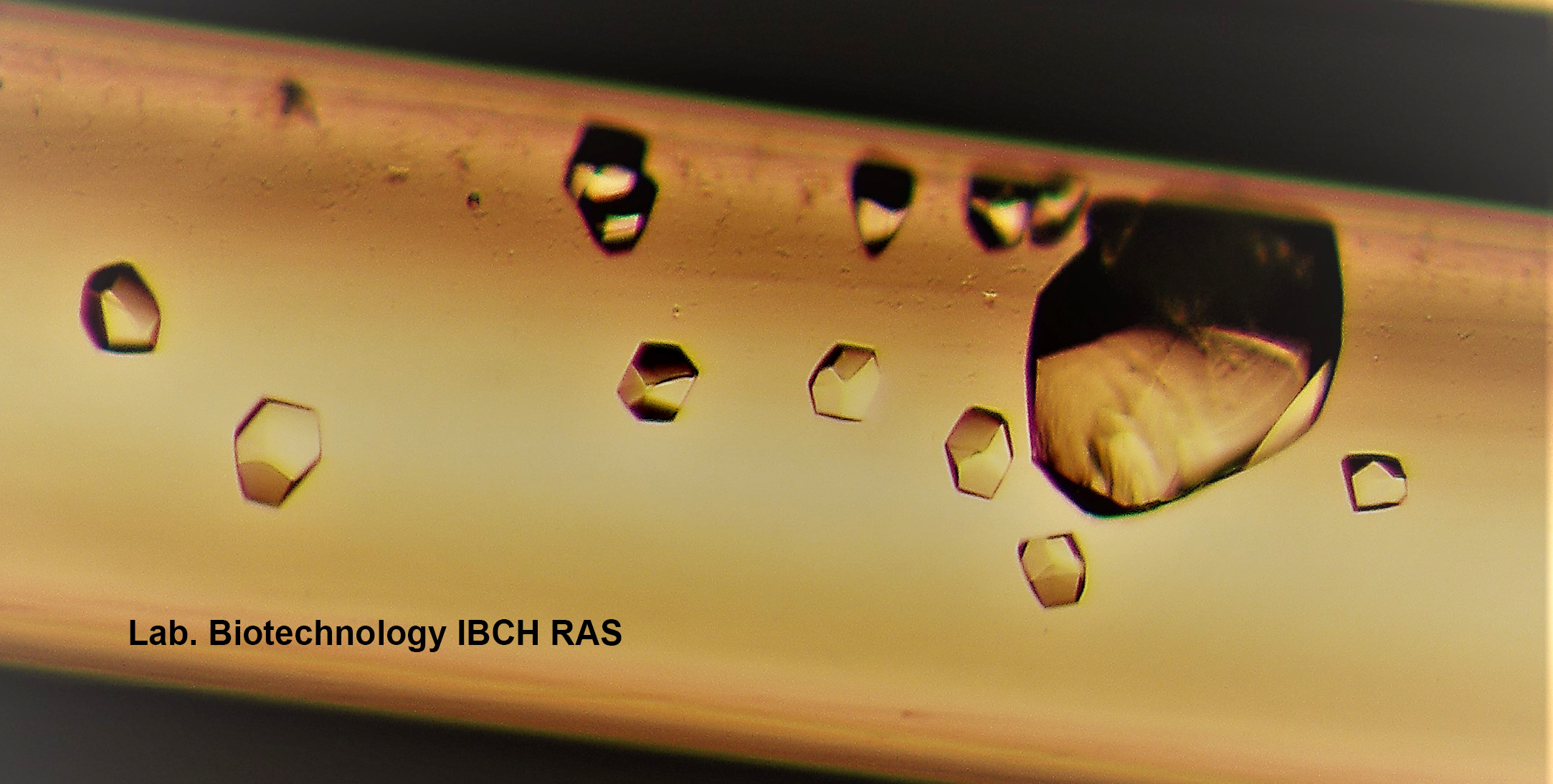
Phosphopanthetein-adenylyltransferase crystals M.tuberculosis
- The crystal structure of the mycobacterial phosphopanthetein-adenylyltransferase complex with substrates was solved by the method of X-ray structural analysis;
- Crystal of the phosphopanthetein-adenylyltransferase M.tuberculosis with ATP
- Crystal of the phosphopanthetein-adenylyltransferase M.tuberculosis with Coenzyme A
- Crystal of the phosphopanthetein-adenylyltransferase M.tuberculosis with DPCoA
- A producer strain of the proteins from the culture filtrate of Mycobacterium tuberculosis, whose family includes the secretory antigens ESAT-6 and CFP10, has been created. Methods for isolating the CFP10-ESAT6 fusion protein have been developed.
Antithrombin field (anticoagulants):
- An intein-mediated biotechnological approach of the production of recombinant analogues of a number of highly specific inhibitors of thrombin from the bloodsucking organisms was developed: hirudin analogues from the medicinal leech Hirudo medicinalis, anophelin from the malaria mosquito Anopheles albimanus, haemadin from Haemadipsa sylvestris, and variegin from the mite Amblyomma variegatum, not requiring the use of specific proteases.
- A comprehensive study of the antithrombotic activity of recombinant anticoagulants was carried out, including: the determination of the antithrombotic activity in vitro using an amidolytic test; the effect of drugs on the clotting time of human blood plasma (thrombin, prothrombin, and activated partial thromboplastin clotting times); pharmacodynamic effects were established when administered intravenously to mice of the CD-1 line. To study the specific antithrombotic activity in vivo, models of tail bleeding and venous thrombosis against the background of hypercoagulation in Sprague Dawley rats were used. Recombinant hirudin-1 (desirudin), unfractionated heparin and dabigatran etexilate (Pradaxa, Boehringer Ingelheim, Germany) were used as reference agents.
Nucleic metabolism enzymes:
- The producer strain of purine nucleoside phosphorylase Thermus thermophilus HB27, phosphoribosyl pyrophosphate synthetase II from T.thermophilus HB27, adenine phosphoribosyltransferase from T.thermophilus HB27, ribokinase from Thermus sp., ribomutase from T.thermophilus HB27, hypoxanthine-guanine phosphoribosyltransferase from T.thermophilus HB27, purine nucleotide phosphorylase from E.coli, phosphoribosyl pyrophosphate synthetase from E. coli, thymidine phosphorylase from E. coli, uridine phosphorylase from E. coli and Salmonella typhimurium, adenosinedeaminase from E. coli, ribokinase and ribomutase from E. coli were obtained.
- Effective methods for the production of nucleic acid enzymes on an industrial scale have been developed.
- A new biosynthesis strategy for biologically important nucleosides based on the multienzyme cascade transformation of D-pentoses into purine nucleosides has been proposed. Threaded synthesis involves sequential transformation into "one-pot» D-pentose 5-phosphate by the action ribokinase, which is then synthesized 1-phosphate peptose under ribomutase, and then condensation of the latter with heterocyclic bases, in the presence of purine and pyrimidine nucleoside phosphorylases gives the desired nucleosides .
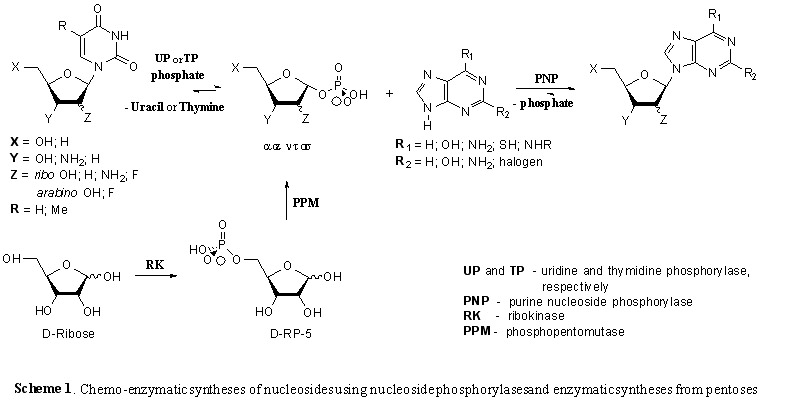
- A new biosynthesis strategy for biologically important nucleotides based on the multienzyme cascade transformation of D-pentoses into purine nucleosides has been proposed. Cascade synthesis involves the sequential transformation of D-pentoses into 5-phosphates in a “single flask” under the action of ribokinase, from which 5-phospho-α-D-pentofuranozo-1-pyrophosphates are then synthesized under the action of phosphoribosyl pyrophosphate synthetase (PRPP-synthetase), the latter condensing heterocyclic purine bases in the presence of adenine phosphoribosyl transferase (APR-transferase) gives the desired nucleotides.
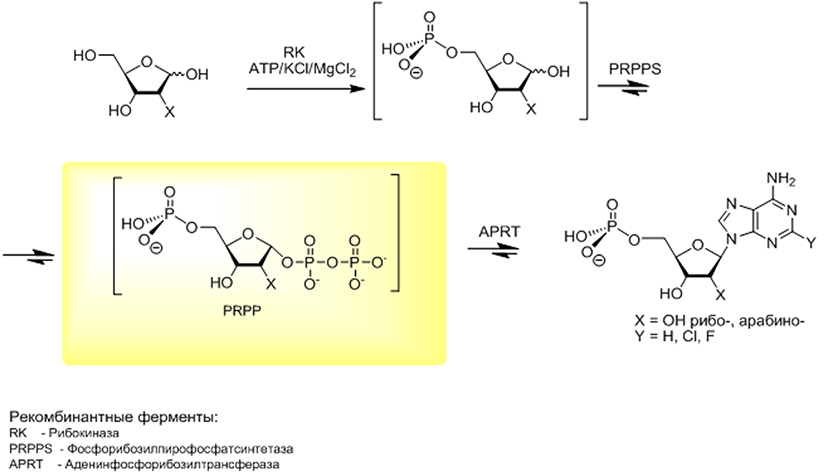
- By the method of counter diffusion in capillaries, crystals of PRPP-synthetase and APR-transferases from E.coli and T.thermophiles, purine nucleoside phosphorylase from E. coli and T.thermophiles, thymidine phosphorylase E. coli including inhibitors and substrates were obtained.
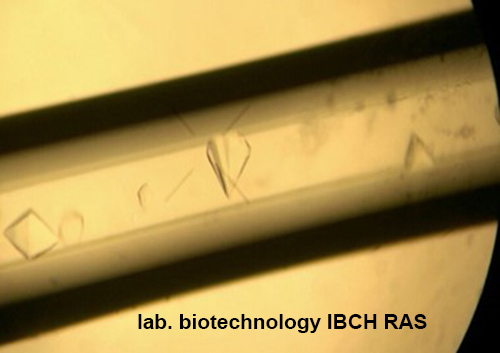
Crystal of PRPP-synthetase from Thermus thermophilus HB27

Crystal of purine nucleoside phosphorylase from E. coli
- The substrate properties of a variety of nucleic acid metabolism enzymes have been determined.
- The crystal structures of the following enzymes in apo-form and with inhibitors and substrates were solved by X-ray diffraction analysis
- Ribokinase from T.sp.
- Adenine phosphoribosyl transferase from T.thermophiles HB27
- Phosphoribosyl pyrophosphate synthetase II from T.thermophilus HB27
- Purine nucleoside phosphorylase from E. coli
- Purine nucleoside phosphorylase from E. coli with 7-deaza-hypoxanthine
- Purine nucleoside phosphorylase from E. coli with Acycloguanosine
- Phosphoribosyl pyrophosphate synthetase II from E. coli
- Thymidine phosphorylase from E. coli
- Thymidine phosphorylase from E. coli with 3’-azido-3’-3’-deoxythymidine
- Thymidine phosphorylase from E. coli with 3'-azido-2'-fluoro-dideoxyuridine
Biopharmaceutical protein and peptide
Peptide hormones
- Human glucagon
- Human interleukin-3
- Human interleukin-15
- Human oxytocin
- Pig and human oxyntomodulin
- Human Proinsulin and C-peptide
- Human tymosine α1
- Human thymosin β4
- Human epidermal growth factor
Anti-and angiogenic proteins
- Pigastin (fragment 24-57 human pigment epithelium-derived factor PEDF)
- Vascular endothelial growth factor: isoform VEGF165b
- Vascular endothelial growth factor VEGF165
- Tumastin (fragment of human tumstatin (L69K-95)
- Endostatin (fragment 1-49)
Antithrombotic drugs
- Anophelin from Anopheles albimanus
- Hirudin-1 from Hirudo medicinalis
- [Leu1, Thr2]-hirudin-1 from Hirudo medicinalis
- Haemadin from Haemadipsa sylvestris
- Variegin from Amblyomma variegatum
Allergen
Phl p I from Phleum pretense
Amb a 1.1 from Ambrosia artemisiifolia
Bet v 1 from Betula pendula
Der p 3 from Dermatophagoides pteronyssinus
Therapeutic peptide
- Cyclotide
- Exendin-4
- ESAT-6 and CFP10-ESAT-6
- Azurin from Pseudomonas aeruginosa and its fragments
- APCH 3 from Sebae anemone (Heteractis crispa)
- Purotoxin-1 from Alopecosa marikovskyi
Nucleic acid enzymes
1. From mesophilic microorganisms
- Adenosine deaminase from E.coli
- Adenine phosphoribosyltransferase from E.coli
- Purine nucleoside phosphorylase from E.coli
- Purine nucleoside phosphorylase mutant Met62Val from E.coli
- Ribose phosphate pyrophosphokinase (RPPK) from E.coli
- Ribokinase from E.coli
- Thymidine phosphorylase from E.coli
- Uridine phosphorylase from E.coli
- Uridine phosphorylase from Salmonella typhimurium
- Phosphopentomutase from E.coli
- Phosphoribosyl pyrophosphate synthetase (PRPPS) from E.coli
- From mesophilic microorganisms
- Adenine phosphoribosyltransferase from Thermus thermophilus HB27
- Hypoxanthine-guanine phosphoribosyltransferase from Thermus thermophilus HB27
- Purine nucleoside phosphorylase I from Thermus thermophilus HB27
- Purine nucleoside phosphorylase II from Thermus thermophilus HB27
- Rybokinase from Thermus specials
- Phosphentomutase from Thermus thermophilus HB27
- Phosphoribosyl pyrophosphate synthetase (PRPPS) from Thermus thermophilus HB27
Enzymes
- Alkaline phosphatase from E.coli
- TEV protease Tobacco Etch Virus
- Human Immunodeficiency Virus (HIV) Protease
- Herpes simplex virus (HSV I) thymidine kinase
Tuberculosis theme
- Aspartate aminotransferase AspC from Mycobacterium tuberculosis
- Imidazole-glycerol-phosphate dehydratase (hisB) from Mycobacterium tuberculosis
- Acid phosphatase from Mycobacterium tuberculosis
- Ketol-acid reductoisomerase from Mycobacterium tuberculosis
- Phosphopanthetein-adenylyltransferase from Mycobacterium tuberculosis
- Ser / Thr-Specific Protein Kinase from Mycobacterium tuberculosis
- Gene product 1606 from Mycobacterium tuberculosis
Biotechnology work
The group carries out a full cycle of works in genetic engineering and protein chemistry ‒ from choosing the gene cloning strategy and its chemical enzymatic synthesis to developing a method for isolation, purification, complete physical and chemical identification and research of biological activity, scaling of the fermentation stage (from 250 ml to 200 l) and protein purification (from 1 mg to 10 g), and also the creation of laboratory and pilot manufacturing technology protocols
| Fullname | Position | Contacts |
|---|---|---|
| Roman Esipov, D.Sc | Head of lab., pr. r. f. | esipov@ibch.ru, +7(495)336-68-33 |
| Roman Esipov, D.Sc | Head of lab., pr. r. f. | esipov@ibch.ru, +7(495)336-68-33 |
| Yulia Abramchik | j. r. f. | |
| Maria Kostromina | r. f. | |
| Evgeny Zayats | j. r. f. | |
| Andronova V.L. | res. eng. | |
| Andrei Karanov | t. q. - lab. as. | |
| Pishko A.S. | t. q. - lab. as. | |
| Ptushkin V.A. | t. q. - lab. as. | |
| Aleksandra Sharafutdinova | t. q. - lab. as. | |
| Torskaya K.I. | t. q. - lab. as. | |
| Antonov K.V. | eng. | |
| Kosarev S.A. | eng. | |
Previously worked here | ||
| Murav'eva T.I., Ph.D. | ||
| Vasiliy Stepanenko, Ph.D. | ||
| Zhukova O.S. | ||
| Chupova L.A., Ph.D. | ||
| Bogdanova A.S. | ||
| Dmitry Makarov, Ph.D. | ||
| Dmitry Lykoshin | ||
 Loading...
Loading...Scientific projects
 Loading...
Loading... Loading...
Loading...
