Laboratory of molecular bases of embryogenesis
The Laboratory studies the molecular-genetic mechanisms of early nervous system development and evolution, and also regeneration of big body appendages on the lower vertebrate model.
One of the projects is devoted to monogenic family of homeobox genes Anf in vertebrates (Dev Biol 1992, 152, 373-382; Development 1995, 121, 3839-3847; Gene 1997, 200, 25-34). For the first time it was found that this gene presents exclusively in the vertebrates genomes (including humans) and absents in invertebrates. It was demonstrated that Anf monitors the development of a unique part of the vertebrates brain – the telencephalon – by inhibiting there the expression of genes that normally control development of the posterior parts of the brain (Development 1999, 126, 4513- 4523; Gene 2002, 285, 279.. -286; Development 2004, 131, 2329-2338; Mech Dev (2004) 121, 1425-1441; Dev Biol 2007; 307, 483-497). Known mutations in this gene in mice and humans have the recessive character, but in the homozygous state lead to the serious abnormalities in brain development, ranging from the underdevelopment of the pituitary gland and optic nerve dysplasia and walls of the cerebral hemispheres to the whole lack of these structures. Thus these results allowed us to hypothesized that the appearance of Anf in vertebrates ancestry could serve as one of the key prerequisites for the final appearance of the telencephalon in evolution (Dev Biol 2007; 307, 483-497). Our recent finding of Anf in the representatives of the most ancient group of modern vertebrates, i.e. in jawless (lampreys), and demonstration that it plays in lamplrey the same function as in other vertebrates, confirm this hypothesis (Sci Rep.. 2016 Dec 23;6:39849)
Also the Laboratory studies the role of genes that were lostg during the vertebrate evolution in the regulation regeneration. We determined that some of the Anf homeobox target genes, namely, the gene of secreted disulphide isomerase Ag1 and genes of small GTPases Ras-dva1, present only in the genomes of lower vertebrates, fish and amphibians, but absent in higher vertebrates, reptiles, birds and mammals (Gene Expr Patterns, 2003, 325-30; Development, in 2006, 133, 485-494; Nucleic Acids Res, 2006, 34, 2247-2257; Gene Expr Patterns, 2011, 11, 156-161). Interestingly, we have demonstrated that besides regulation of regeneration, Ag1 and Ras-dva1 regulate another important process – the telencepalon development (Sci Rep, 2013, 3, 1279; Biol Open, 2014, 3, 192-203; Sci Rep, 2015, 5: 8123). Therefore we hypothesized that the disappearance of these and probably some other genes in vertebrate ancestry could be a kind of trade of between the reduced regenerative capacity and the opportunity of the progressive development of the telencephalon. Taking in mind this hypothesis, together with our colleagues from the Institute for Information Transmission Problems (Prof. A.V. Lyubetsky lab.) we developed bioinformatics program that allows identify genes that have arisen or disappeared at a specified stage of evolution. The functions of some genes found by this program are studying in the Laboratory now.
A study of gene network associated with the Anf gene functioning in the cells of the telencephalon rudiment lead to the discovery of a number of previously unknown genes that play an important role in embryogenesis. For example, we identified and studied regulators of the early brain development – secreted proteins Noggin2 and Noggin4 (Gene Expr Patterns, 2006 6:180-6; Development, 2011, 138, 5345-5356; Int J Dev Biol 2012; 56: 403-6; Sci Rep 1996, 14, 6:23049). In contrast to their commonly known homolog, an inhibitor of the BMP – protein Noggin1 these proteins inhibit the function of the regulator of the posterior regions of the central nervous system – the secreted protein Wnt8.
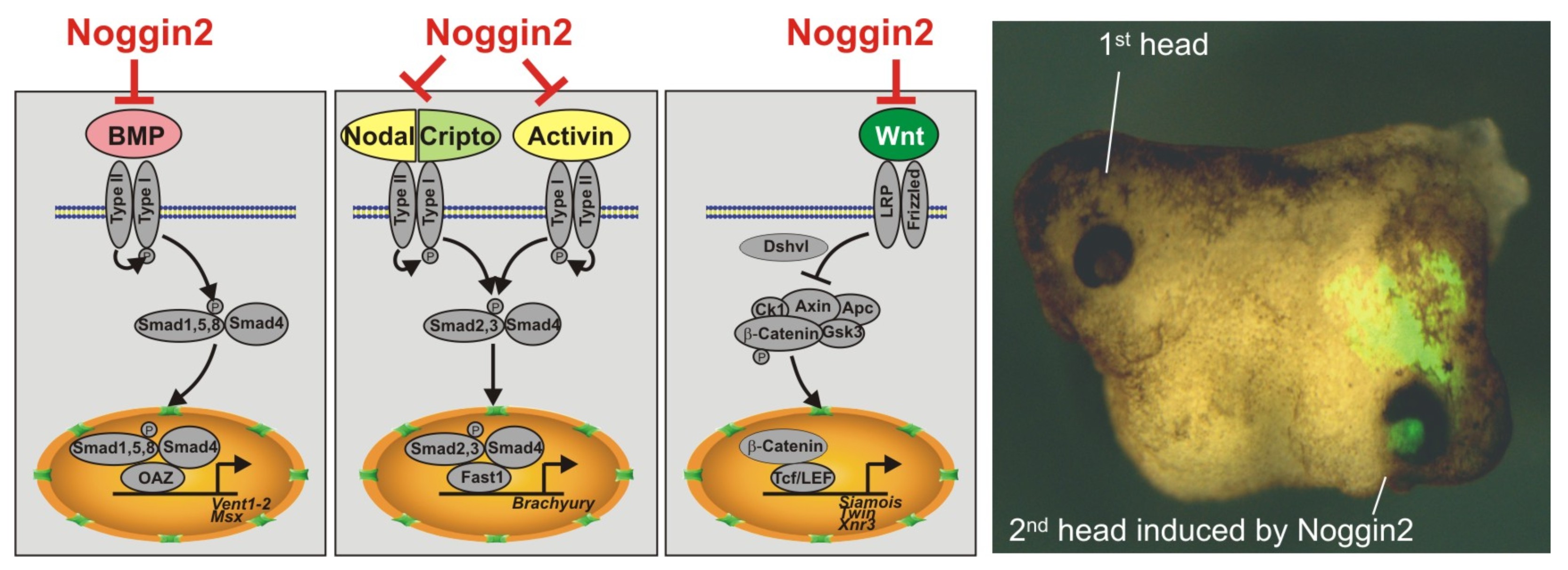
Ectopically expressed Noggin2, which inhibits three signaling cascades, BMP, Activin/Nodal and Wnt, is sufficient for the induction of a secondary head on the ventral side of the Xenopus laevis embryo.
In addition, we have developed a number of methods for the study of diffusion and interactions of secreted morphogen proteins in the intercellular space of embryonic tissues (Sci Rep, 2016 6:23049; Biochem Biophys Res Commun, 2015; 468:331-6). For the first time the diffusion coefficients of Noggin2 and 4 and Wnt8 in vivo protein families were measured and the role in their diffusion of the adsorption on the extracellular matrix was demonstrated. Also, we have shown with the mathematical modeling that the adsorption may be an important factor needed to create spatial dynamic structures during embryogenesis (PLoS One. 2017 Feb 7;12(2):e0171212. doi: 10.1371).
Also we have studied during the early development of the central nervous system the role of the cytoskeletal protein Zyxin. This protein is able to binding with Anf homeodomain protein as well as with the effector of Shh signaling cascade – Gli1 transcription factor, inhibiting their activity (Dev Dyn, 2008, 237, 736-749; Dev Biol, 2013, 380, 37-48). The findings are important because they demonstrate for the first time a link between one of the cytoskeletal regulatory proteins that control cells morphogenetic movements and mechanical tensions in the developing embryo and an important signaling pathway, that regulates cell differentiation during early development of the central nervous system. Now we studying the role of Zyxin in regulation of other pathways and also the role of mechanical tensions in gene expression during embryogenesis (Genesis. 2017 Feb 25. doi: 10.1002/dvg.23026)
The Laboratory is also working on testing the possibility of using genetically encoded fluorescent reporters and sensors for studying various processes in early embryogenesis and regeneration (Nat Biotechnol, 1999, 17, 969-973; Science, 2000, 290: 1478-1479; Nat Biotechnol, 2003 , 21: 191-194; Nat Methods, 2007, 4, 741-746; Nat Methods, 2010, 7, 827-829; Nat Commun, 13, 2012; 3:1204). In particular, for the first time the possibility of using fluorescent proteins from coral polyps for in vivo monitoring of cells in Xenopus embryos was demonstrated (Nat Biotechnol, 1999, 17, 969-973).
Aquarium room, in which frogs and tadpoles of some transgenic lines, as well as adult frogs expressing fluorescent proteins under the control of promoters of various genes are kept.
The Laboratory collaborates with other laboratories of the Institute, as well as with the Department of Biophysics of the Moscow State University, the Department of Embryology of the Moscow State University, the Center "Bioengineering" RAS, the Belozersky Institute of Physico-Chemical Biology of the Moscow State University, the Institute for Information Transmission Problems RAS, Kurchatov Institute, Massachusetts Institute of Technology (USA), the University of Virginia (USA).
The Laboratory was founded in 2005 by a group of the same name released in 1995 from the Laboratory of structure and function of human genes.
- Study the molecular genetic mechanisms of early development and evolution of the nervous system.
- Study and modeling of the morphogens diffusion in the embryonic tissues.
- Study of the processes of the large body appendages regeneration on lower vertebrate models.
- Creating and maintaining lines of transgenic Xenopus frogs, expressing genetically encoded fluorescent reporters and sensors.
- Anf/Hesx1 homeobox gene was identified for the first time in different vertebrates, including human. It was shown that this gene present only in vertebrates. The hypothesis of the role of Anf/Hesx1 as the primary factor that initiated emergency of the telencephalon in vertebrates was proposed.
- It is found that some of the target genes of Anf homeobox gene (Ag1, Ras-dva1) present only in the genomes of lower vertebrates, fish and amphibians, but absent in higher vertebrates, reptiles, birds and mammals. It was shown that in lower vertebrates these genes are involved both in regulation of the telencephalic development and the body appendages regeneration. It was hypothesized that disappearance of these and probably some other genes in vertebrate ancestry could be a kind of trade of between the reduced regenerative capacity and the opportunity of the progressive development of the telencephalon.
- Early brain development regulators – secreted proteins and Noggin2 Noggin4 – were identified and studied.
- A number of methods for the studing of diffusion and interactions of secreted morphogens in the intercellular space of embryonic tissues were developed. By these methods, diffusivities of some morphogens were measured in vivo.
- For the first time the role of Zyxin cytoskeletal protein in the early embryonic development was studied. In particular, it was shown that Zyxin is able to binding with Anf homeodomain protein and with the effector of Shh signaling cascade – the transcription factor Gli1 and inhibit their activity.
- The technology of two-color reporter vector, significantly increases the efficiency of the functional analysis of the promoter, with the ability to compare the expression of two potential deletion mutants studied gene promoter in the same transgenic embryos was developed (Development, 2004, 131, 2329-2338).
- Using genetically encoded SypHer2 pH-indicator on the tadpole Xenopus model was discovered a previously unknown effect – the rapid acidification of the cells cytoplasm near the site of the tail amputation (Biochim Biophys Acta, 2015 1850:2318-28). This effect is one of the first reactions of the organism to amputation, and may be important for subsequent regeneration.
| Fullname | Position | Contacts |
|---|---|---|
| Andrey Zaraisky, Prof., D.Sc | Head of lab., pr. r. f. | |
| Andrey Zaraisky, Prof., D.Sc | Head of lab., pr. r. f. | |
| Andrey Bayramov, D.Sc | l. r. f. | |
| Fedor Eroshkin, Ph.D. | s. r. f. | xenopus.fe@gmail.com |
| Natal'ja Martynova, Ph.D. | s. r. f. | martnat61@gmail.com, +7(916)1811632 |
| Galina Ermakova | r. f. | |
| Evgeny Orlov, Ph.D. | r. f. | |
| Elena Parshina, Ph.D. | r. f. | |
| Maria Tereshina, Ph.D. | r. f. | |
| Timoshina P.S. | j. r. f. | |
| Bannikova M.A. | res. eng. | |
| Volkov S.A. | res. eng. | |
| Kolyupanova N.M. | t. q. - lab. as. | |
| Kurdyumov A.M. | t. q. - lab. as. | |
| Serebryakova M.V. | eng. | |
| Shitikov A.D. | j. r. f. | |
| Vatanina N.I. | eng. | |
Previously worked here | ||
| Anastasiya Ivanova, Ph.D. | ||
| Alexey Nesterenko | ||
| Korotkova D.D., Ph.D. | ||
| Alexandr Borodulin, Ph.D. | ||
| Araslanova K.R. | ||
| Ivanova E.D. | ||
| Karpova L.A. | ||
| Aver'yanova O.V. | ||
| Solovieva E.A. | ||
 Loading...
Loading...Scientific projects
 Loading...
Loading...Andrey Zaraisky
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
Scopus: 7003375442, ORCID: 0000-0003-4681-8169
 Loading...
Loading...
