Лаборатория молекулярной токсинологии
Лаборатория проводит фундаментальные исследования по изучению молекулярной структуры компонентов животных ядов и механизмов их действия. В качестве основного инструмента для исследований механизмов функционирования нервной системы и проведения нервных импульсов используются токсины. В результате расширения круга интересов и задач Лаборатории ее сотрудники изучают яды, которые не только воздействуют на нейрорецепторы, но и влияют на гемостаз.
Лаборатория молекулярной токсинологии сотрудничает с рядом зарубежных лабораторий и групп. Так, изучением различных рецепторов и поиском новых токсинов она занимается совместно с Рурским университетом (Ruhr-Universität Bochum, Германия). Кроме того, исследование токсинов проводится совместно с белорусскими учеными («Институт физиологии НАН Беларуси»), которые изучают влияние нейроактивных соединений ядов на развитие опухолей (в качестве модельной опухоли используется карцинома Эрлиха). Особый интерес представляет сотрудничество Лаборатории со специалистами из Вьетнама (Institute of Applied materials science, Хошимин), вместе с которыми изучаются яды вьетнамских животных. Также Лаборатория молекулярной токсинологии налаживает связи с учеными из Индии.
Помимо традиционно изучаемых нейротоксинов Лаборатория исследует пептиды и белки, влияющие на свертываемость крови. Ученые занимаются установлением их структуры и определением биологических свойств. Кроме того, сотрудники Лаборатории вместе с коллегами из филиала ИБХ (г. Пущино) изучают снижающие кровяное давление пептиды, которые были найдены в яде бирманской гадюки Azemiops feae. Отдел молекулярных основ нейросигнализации занимается доклиническими исследованиями одного из пептидов этой ядовитой змеи, который взаимодействует с холинорецепторами и позиционируется как местный миорелаксант. В будущем это позволит использовать его для лечения ряда заболеваний, в которых существует необходимость в местном расслаблении мышц.
Лаборатория молекулярной токсинологии была создана в 2009 году. Вместе с Лабораторией лиганд-рецепторных взаимодействий она входит в состав Отдела молекулярных основ нейросигнализации, который, в свою очередь, возник вследствие расширения круга задач существовавшей до этого Лаборатории рецепции нейропептидов. Имеющиеся у обеих Лабораторий взаимодополняемые методы структурной нейробиологии помогают сотрудникам проводить совместные исследования и наиболее эффективно выполнять главные задачи отдела.
- Проведение фундаментальнх исследований по изучению молекулярной структуры компонентов животных ядов и механизмов их действия.
- Исследование пептидов и белков, влияющих на свертываемость крови, установление их структуры и определение биологических свойств.
В результате работы выделено и охарактеризовано более трех десятков новых белков, обладающих уникальными структурными и функциональными характеристиками.
- При изучении яда кобры Naja kaouthia впервые обнаружено несколько новых токсинов. Так, выделен и охарактеризован первый и пока единственный гликозилированный трехпетельный токсин - цитотоксин 3. Установлено, что гликозилирование этого токсина приводит к существенному снижению его цитотоксичности. Также впервые показано, что давно известные «слабые» токсины способны взаимодействовать с никотиновыми холинорецепторами. При этом, обладая слабым сродством к рецептору, такие токсины связываются практически необратимо. Кроме того, эти токсины способны также аллостерически связываться с мускариновыми холинорецепторами. В яде этой же кобры впервые обнаружены ковалентные димеры трех-петельных токсинов. Показано, что такая димеризация вызывает изменение биологической активности токсинов, образующих димер.
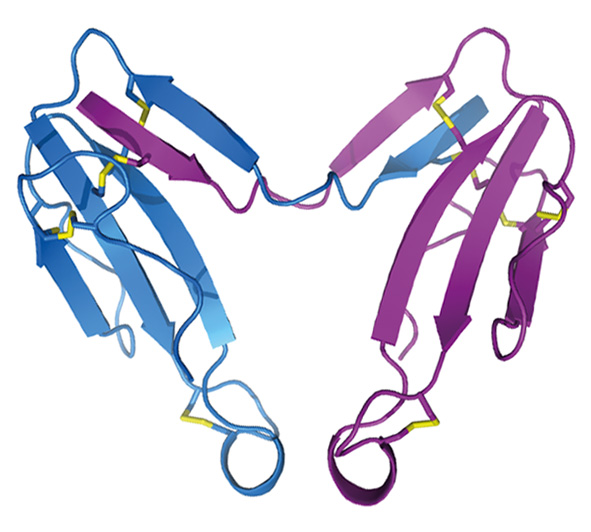
Кристаллическая структура димера альфа-кобратоксина (Osipov et al., (2012) J. Biol. Chem. 287(9), 6725-6734.)
- Несколько нейротоксичных белков выделено из ядов гадюк. Так, из яда гадюки Никольского выделена гетеродимерная фосфолипаза А2, проявляющая пресинаптическую нейротоксичность. Из яда африканской шумящей гадюки Bitis arietans выделен блокирующий никотиновый холинорецептор (нХР) белок с молекулярной массой 27,4 кДа. N-концевая последовательность этого белка, названного битанарином, гомологична N-концевым аминокислотным последовательностям фосфолипаз А2 из ядов змей, имеющих молекулярную массу около 14 кДа и не обладающих способностью блокировать холинорецепторы. С использованием метода радиолигандного анализа установлено взаимодействие битанарина с нХР мышечного и альфа7 типа, а также с ацетилхолин-связывающими белками моллюсков L. stagnalis и A. californica. По своей активности битанарин близок так называемым необычным или слабым трехпетельным токсинам из ядов кобр. Этот белок обладает также фосфолипазной активностью, сопоставимой с активностью фософлипаз А2 яда кобр. Битанарин представляет собой фосфолипазу А2 нового типа и является первой фософлипазой, для которой показано взаимодействие с нХР.
- При исследовании компонентов ядов, воздействующих на гемостаз, также обнаружен ряд новых белков. Так, из яда кобры Naja oxiana выделена металлопротеиназа оксиагин, для которой впервые показан новый механизм ингибирования системы комплемента. В яде кобры Naja haje обнаружена фософлипаза А2, обладающая уникальной способностью ингибировать тромбин. Это – первый представитель нового структурного типа белковых ингибиторов тромбина.
| ФИО | Должность | Контакты |
|---|---|---|
| Уткин Юрий Николаевич, д.х.н. | зав. лаб., г.н.с. | utkin@ibch.ru |
| Дьяченко Игорь Александрович, д.б.н. | с.н.с. | |
| Осипов Алексей Валерьевич, к.х.н. | с.н.с. | |
| Шайхутдинова Эльвира Рауильевна, к.б.н. | с.н.с. | |
| Старков В.Г. | н.с. | |
| Исмаилова Алина Магомедовна | инж.-иссл. | |
| Северюхина Мария Сергеевна | инж.-иссл. | |
| Сухов Д.А. | инж.-иссл. | |
| Махтумова М. | инженер | |
| Тимофеев Н.Д. | инженер | |
Ранее здесь работали | ||
| Шелухина Ирина Валерьевна, д.х.н. | shelukhina.iv@yandex.ru | |
| Аверин А.С. | ||
| Синявин Андрей Эдуардович, к.х.н. | ||
| Бороздина Наталья Андреевна | ||
| Кост В.Ю. | ||
| Паликов Виктор Анатольевич | ||
| Паликова Юлия Александровна | ||
| Рамазанова А.С. | ||
| Андреева Т.В. | ||
| Казаков О.В. | ||
| Рашимова А.Д. | ||
| Горбачева Е.И. | ||
| Афанасьева С.О. | ||
| Гарифулина А.И. | ||
| Епифанова Л.А. | ||
| Мещеряков П.А. | ||
| Спирова Е.Н., к.б.н. | ||
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Уткин Юрий Николаевич
Москва, ул. Миклухо-Маклая, 16/10 — На карте
31/606
Scopus: 35598587900, ORCID: 0000-0002-4609-970X, ResearcherID: F-1958-2014, Google Scholar
 Загрузка...
Загрузка...
