Press-room / news / Science news /
«A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance»
Laboratory of Genomics of Adaptive Immunity of IBCh RAS employees M. Shugay, E. Putintceva and D. Chudakov participated in the internationally renowned immunologist Alexander Rudensky the research of the mechanisms for expansion of regulatory T-cell repertoire and its role in self-tolerance. The article with results of research was published in the Nature.
T-cell receptor (TCR) signalling has a key role in determining T-cell fate. Precursor cells expressing TCRs within a certain low-affinity range for complexes of self-peptide and major histocompatibility complex (MHC) undergo positive selection and differentiate into naive T cells expressing a highly diverse self-MHC-restricted TCR repertoire. In contrast, precursors displaying TCRs with a high affinity for ‘self’ are either eliminated through TCR-agonist-induced apoptosis (negative selection) or restrained by regulatory T (Treg) cells, whose differentiation and function are controlled by the X-chromosome-encoded transcription factor Foxp3.
Foxp3 is expressed in a fraction of self-reactive T cells that escape negative selection in response to agonist-driven TCR signals combined with interleukin 2 (IL-2) receptor signalling. In addition to Treg cells, TCR-agonist-driven selection results in the generation of several other specialized T-cell lineages such as natural killer T cells and innate mucosal-associated invariant T cells. Although the latter exhibit a restricted TCR repertoire, Treg cells display a highly diverse collection of TCRs.
A. Rudensky research group (include Laboratory of Genomics of Adaptive Immunity of IBCh RAS employees M. Shugay, E. Putintceva, and D. Chudakov ) explore in mice whether a specialized mechanism enables agonist-driven selection of Treg cells with a diverse TCR repertoire, and the importance this holds for self-tolerance. Researchers show that the intronic Foxp3 enhancer conserved noncoding sequence 3 (CNS3) acts as an epigenetic switch that confers a poised state to the Foxp3 promoter in precursor cells to make Treg cell lineage commitment responsive to a broad range of TCR stimuli, particularly to suboptimal ones. CNS3-dependent expansion of the TCR repertoire enables Treg cells to control selfreactive T cells effectively, especially when thymic negative selection is genetically impaired. Our findings highlight the complementary roles of these two main mechanisms of self-tolerance.
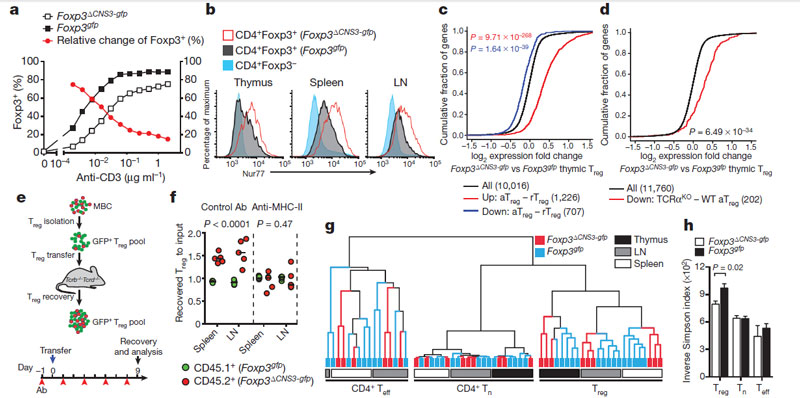
CNS3 shapes the Treg cell repertoire
Read more: «A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance», Nature (2015).
december 1, 2015

