Press-room / news / Science news /
Novel peptide chemistry in terrestrial animals: luciferin analogs from the bioluminescent earthworm Fridericia heliota
Research group from Institute of bioorganic chemistry (IBCh RAS, Moscow) in collaboration with scientists from Institute of biophysics (IBP SB RAS, Krasnoyarsk) presents new findings as part of the research project on earthworm bioluminescent system. Results published in Chemistry — A European Journal document structure elucidation of a set of natural analogs of Fridericia heliota luciferin [1].
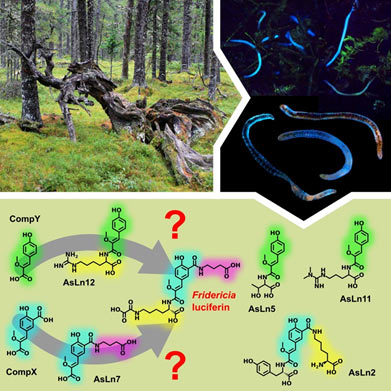
Cover picture
The exploration of luminescence phenomenon in live animals, a so-called “cold light”, started as early as in 19th century. According to present knowledge, 30 different mechanisms of bioluminescence exist, of which the luciferin structures were fully determined only for seven, while the structure of the 8th luciferin from Fridericia heliota was established in 2014 by joint efforts of the specialists in organic synthesis from IBCh RAS and researchers from IBP SB RAS [2, 3]. In the wake of a novel luciferin discovery the inquisition of its biosynthetic pathway immediately becomes the first priority.
Currently the group led by Ilia Yampolsky is conducting a detailed investigation of Fridericia heliota bioluminescent system. The extract of earthworm biomass contained a number of luciferin analogs, designated AsLn5, AsLn7, AsLn11 and AsLn12. In the course of purification several dozens of micrograms of each compound were obtained, which permitted the determination of their chemical structures, using modern methods of NMR and mass spectroscopy. It was shown that the luciferin analogs are unusual peptides composed of residues of tyrosine modifications (CompX and CompY) and γ-aminobutyric acid, threonine, homoarginine or asymmetric dimethylarginine.
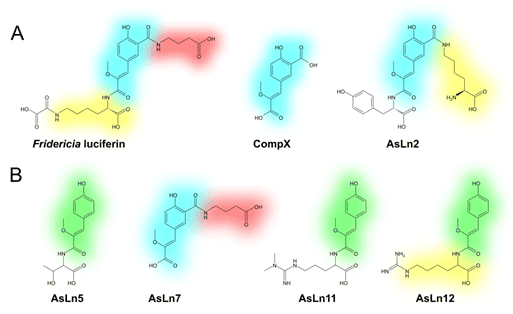
Figure 1. Chemical structures of Fridericia luciferin and its analogs with modified tyrosine core CompX (blue) and CompY (green).
Modified tyrosine core CompX serves as a light emitter of Fridericia luciferin (fig. 1). NMR and UV spectral data of luciferin analogs (fig. 2) showed that AsLn7 contains the same chromophore, whereas the other three compounds include a different tyrosine modification.
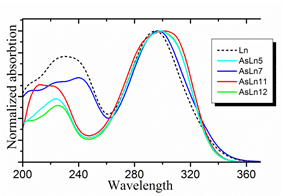
Figure 2. UV spectral data of AsLn5, AsLn7, AsLn11, AsLn12 in comparison with Fridericia luciferin.
New chromophore similarly to CompX was designated CompY. To determine the configuration of the double bond of the new compound both the E- and Z-isomers of CompY were synthesized, and their NMR spectral data compared to those of the natural compound.
Structures of other fragments and their interconnection in compounds AsLn5, AsLn11 and AsLn12 were established by a combination of NMR and mass-spectrometry methods. Interestingly, 1H NMR titration of luciferin analogs permitted to identify the order of peptide bonds connecting the residues (this method was previously employed in course of luciferin structure elucidation [3]). Thus, molecular structures of four unusual natural compounds — members of a new class of animal peptides — were unambiguously determined.
Peptide structures of AsLn5, AsLn7, AsLn11 and AsLn12 are extremely unusual for terrestrial animals. Interestingly, CompX moiety is unique to F. heliota earthworms, whilst CompY, that unlike CompX lacks an aromatic carboxyl group, was reported to be a structural fragment of substances extracted from ascidians [4, 5].
Why is this set of compounds typical to Fridericia heliota? What is the biosynthetic link between AsLns? Do F. heliota worms synthesize AsLns, or do they obtain some key components, like CompX or CompY, through diet or from some unknown sybmionts? All of these questions are tightly connected to the issue of luciferin biosynthesis (fig. 3).
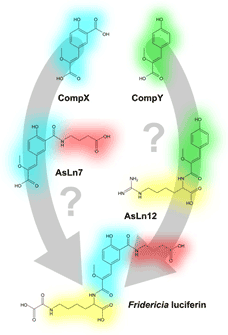
Figure 3. Possible pathways of Fridericia luciferin biosynthesis.
The structure of luciferin implies two possible pathways of biosynthesis: presumably the four fragments of luciferin are enzymatically consecutively linked to each other. This pathway is strongly supported by the structure of AsLn7. Alternatively, luciferin is synthesized in vivo from AsLn12 via carboxylation and replacement of guanidinium fragment in homoarginine by oxalic acid residue. At this point it is uncertain which of these pathways is more probable. Answer to an enigma of the structure of natural analogs of luciferin is merely the initial step on the road to establishing the full biosynthetic pathway of luciferin. Although, for the moment new findings raise more questions, than give answers — the truth is out there.
References
-
Dubinnyi M.A., Tsarkova A.S., Petushkov V.N., Kaskova Z.M., Rodionova N.S., Kovalchuk S.I., Ziganshin R.H., Baranov M.S., Mineev K.S., Yampolsky I.V. (2015). Novel Peptide Chemistry in Terrestrial Animals: Natural Luciferin Analogues from the Bioluminescent Earthworm Fridericia heliota. Chem. Eur. J. 21,
2–8. doi: 10.1002/chem.201406498; - Press release IBCh RAS from 16.04.2014: “Novel luciferin from Siberian bioluminescent worm”;
-
Petushkov V.N., Dubinnyi M.A., Tsarkova A.S. et al. (2014). A novel type of luciferin from Siberian luminous earthworm Fridericia heliota: structure elucidation by spectral studies and total synthesis. Angew. Chem. Int. Ed. 53,
5566–5568; -
Kehraus S., Gorzalka S., Hallmen C., Iqbal J., Müller C.E., Wright A.D., Wiese M., König G.M. (2004). Novel amino acid derived natural products from the ascidian Atriolum robustum: identification and pharmacological characterization of a unique adenosine derivative. J. Med. Chem. 47,
2243–2255; -
Yin S., Cullinane C., Carroll A.R., Quinn R.J., Davis R.A. (2010). Botryllamides K and L, new tyrosine derivatives from the Australian ascidian Aplidium altarium. Tetrahedron Lett. 51,
3403-3405.
february 4, 2015

