Press-room / news / Science news /
Intramolecular Hydrogen Bonding in N6-Substituted 2-Chloroadenosines: Evidence from NMR Spectroscopy
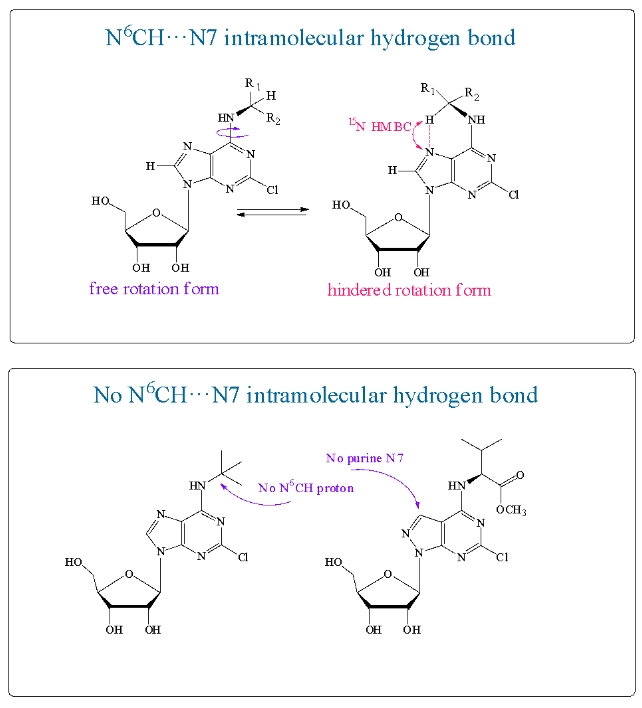
Two forms were found in the NMR spectra of N6-substituted 2-chloroadenosines. The proportion of the mini-form was 11–32% of the main form. It was characterized by a separate set of signals in NMR spectra. The team of scientists from IBCh RAS assumed that the mini-form arises due to an intramolecular hydrogen bond between the N6–CH proton of the substituent and the N7 atom of purine. The 15N-HMBC spectrum confirmed the presence of a hydrogen bond in the mini-form of the nucleoside and its absence in the main form. Compounds incapable of forming such a hydrogen bond were synthesized. In these compounds, either the N7 atom of the purine or the N6–CH proton of the substituent was absent. The mini-form was not found in the NMR spectra of these nucleosides, confirming the importance of the intramolecular hydrogen bond in its formation.
The results are published in the International Journal of Molecular Sciences.
june 14, 2023


