Press-room / news / Science news /
Fractal droplets
Biological polymers (proteins, nucleic acids and their mixtures) are able to form macromolecular complexes in solution, which at a certain saturating concertation of a polymer grow in size and when crossing the diffraction limit (0.25 µm) become visible under the microscope. Such microscopically observed spherical liquid-like formations have various naming depending on a context such as: «coacervate droplets», biomolecular condensates, etc. Although these polymer droplets (especially proteinaceous) gained numerous attentions of scientist during the last century, e. g. as potential «protocells» in «the primordial soup» according to the life-origin hypothesis by Alexander Oparin, active studies of their functional roles in the living cells flourished in the recent decades.
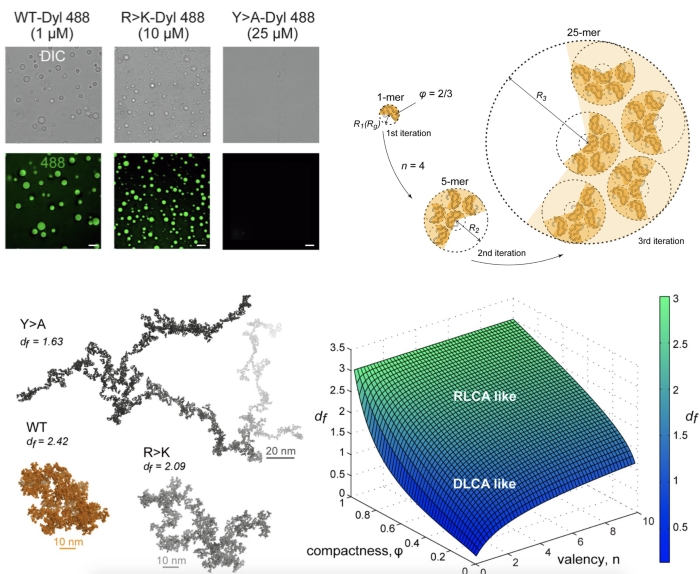
Biomolecular condensates are widely considered as an important mode of subcellular organization in diverse biological contexts (nucleoli, Cajal bodies, stress granules, neuronal granules, P- bodies, and other non-membrane cellular organelles). Intrinsically disordered proteins (IDPs), in particular, feature extensively in many condensates and are thought to contribute to their formation via transient intermolecular contacts (Banani et al., 2017; Uversky, 2021). At the same time, a multiscale picture of the spatial organization and dynamics of IDP condensates, connecting the conformational properties and interaction patterns encoded in the sequences of individual polypeptides with the features of the condensates they build, remains unclear. The joint study by IBCH RAS and University of Vienna presents a newly derived fractal model that provides a quantitative link between the atomistic features of an IDP with the spatial organization of the biomolecular condensate it forms. The proposed model resonates with the emerging concepts of coupling between phase separation and percolation in condensate formation (Mittag & Pappu, 2022).
For an 80-aa IDP fragment of yeast transcriptional regulator Lge1 (Lge11-80 ), the study connects single- and multi-copy, microsecond molecular dynamics simulations with the experimentally observed, micrometer-scale behavior of the protein’s condensate. Specifically, the proposed formalism describes condensate architecture across length-scales as a function of protein valency and compactness. Importantly, simulation-derived fractal dimensions of condensates of Lge11-80 and its mutants are shown to match the in vitro observations. Finally, the formalism provides an atomistic model of a micrometer-size condensate starting from individual simulated protein conformers. The presented modeling framework enables a multiscale description of biomolecular condensates and embeds their study in a wider context of colloid self-organization. The study is published in eLife.
References
- Banani et al., Nat. Rev. Mol. Cell Biol., 2017, 18(5), 285-298. doi:10.1038/nrm.2017.7
- Uversky, V. N. Annu. Rev. Biophys., 2021, 50(1), 135-156. doi:10.1146/annurev-biophys- 062920-063704
- Mittag, T., & Pappu, R. V. Mol. Cell, 2022, 82(12), 2201-2214. doi:10.1016/j.molcel.2022.05.018
september 11, 2023


