Press-room / news / Science news /
Open AccessArticle Galectin-9 as a Potential Modulator of Lymphocyte Adhesion to Endothelium via Binding to Blood Group H Glycan
Adhesion of leukocytes is a key stage in their trafficking into sites of inflammation. This process is mediated through the interaction of integrins, selectins or CD44, while the role of galectins is not completely clear. It is known that galectins are capable of interacting with oligolactosamines of endothelial cells, and also that in vitro tandem-type galectins (Gal-4, -8 and -9) bind with high affinity to glycans of the ABH blood group system. This work shows that in a cell lines system gal-9 mediates lymphocyte adhesion to endothelial cells through binding to their H-glycan, suggesting that lymphocyte adhesion to endothelium in the circulation occurs similarly and is regulated by the level of galectin-9 expression.
Along with integrins, selectins and CD44 of leukocytes, another family of adhesion molecules - galectins (gal), widely present on lymphocytes, could also be potential participants in their adhesion to the endothelium. Galectins bind to oligolactosamines (LNs) found on endothelial cells (ECs). In addition to LN, glycans of the ABH blood group system, which are found in glycolipids and glycoproteins, are strongly expressed on EC. However, there is no information that galectins are involved in adhesion to the endothelium due to binding specifically to ABH glycans, although tandem-type galectins (gal-4, -8 and -9) recognize them with an affinity an order of magnitude higher than LNs in in vitro systems. Gal-8 and gal-9 are detected in lymphocyte glycocalyx; they have two carbohydrate-binding domains (CBDs) - N-CBD and C-CBD. The first exhibits high affinity for LN and is involved in anchoring the lectin on the cell; with the help of the second, galectins bind to glycans of other cells, in particular with ABH glycans. This work investigated whether gal-9 is able to mediate the adhesion of lymphocytes to endothelial cells through binding to their H-glycan. The model system was the interaction of Jurkat cells (lymphocytic origin, expressing gal-9) with the endothelial cell culture EA.hy 926 (expressing H-glycans). The focus of the study was the H antigen, since EA.hy 926 cells lack A- and B-glycosyltransferases. EA.hy 926 cells were defucosylated, then a synthetic lipid construct containing the trisaccharide Fucα1-2Galβ1-4GlcNAcβ (H type 2) as a glycan was inserted into them (Fig. 1).
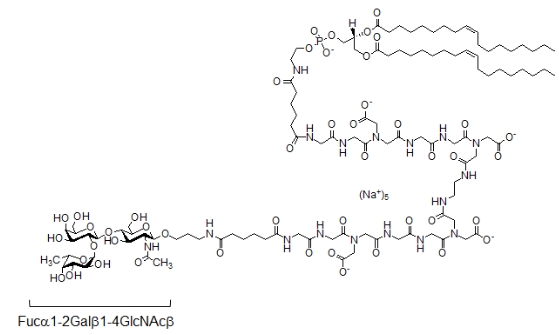
Fig. 1. Structure of synthetic glycolipid H (type 2)
After the insertion, the glycan was detected in the immediate vicinity of the DPH-stained membrane zone and coincided in location with the H-glycan in native cells (Fig. 2).
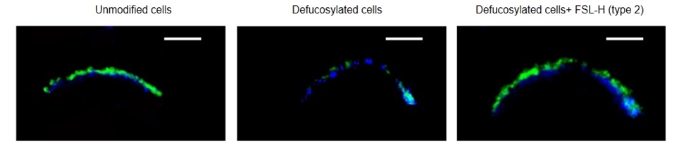
Fig. 2. Localization of H (type 2) in EA.hy 926 cells, confocal microscopy. To stain the membrane, DPH (1,6-diphenylhexatriene, blue) reagent was used, which accumulates and fluoresces only in a hydrophobic environment. N-glycans were detected using biotinylated lectin UEA I plus streptavidin-FITC conjugate (green). Scale=5 µm.
Removal of fucose resulted in decreased binding of free gal-9 to cells. Due to the insertion of H (type 2), the binding of gal-9 to defucosylated cells increased threefold (Fig. 3), and was comparable to that for native cells.
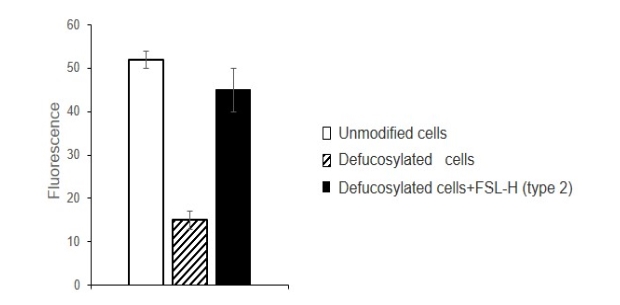
Fig. 3. Gal-9 binding to EA.hy 926 cells, cytofluorimetry. Cells were incubated with fucosidase, then with glycolipid H (type 2). After the embedding and washing, cells were incubated with gal-9, anti-gal-9 antibodies, and secondary anti-IgG-FITC conjugate. The results of cytofluorimetric analysis are presented as the average of three independent experiments, p<0.001.
The presence of trisaccharide H (type 2) turned out to be sufficient for the interaction of gal-9 in Jurkat cells with EA.hy 926 cells. To study intercellular adhesion, Jurkat cells were added to a monolayer of ECs containing different amounts of H-glycan: native, defucosylated and defucosylated with subsequent insertion of H (type 2) into their membrane. After washing, the adherent Jurkat cells were identified due to gal-9 - the lectin-expressing cells were counted. On EA.hy 926 cells treated with fucosidase, only 4% of the amount of Jurkat cells adhered to native cells was detected (Fig. 4 B, D), and on defucosylated cells after insertion of H (type 2) - up to 50% (Fig. 4 C , G).
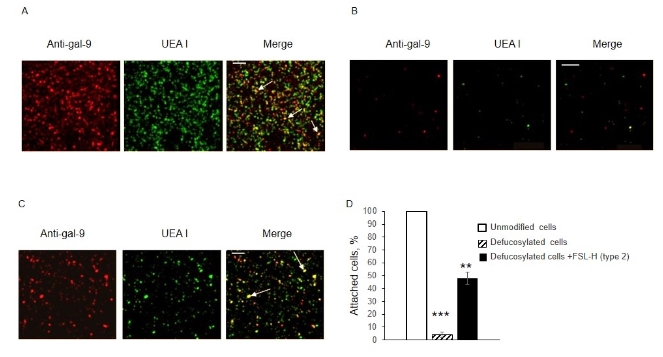
Fig. 4. Adhesion of Jurkat cells to intact (A), defucosylated (B) and defucosylated EA.hy 926 cells after insertion of H (type 2) (C), confocal microscopy. Gal-9 was detected by anti-Gal-9+ IgG-Alexa594 (in red), H (type 2) was detected by biotinylated UEA I + Str-FITC (in green). Jurkat cells adherent to EA.hy 926 are shown with arrows in the images obtained by overlaying (in yellow). Adherent cells counted in three independent measurements are presented as the percentage of adherent to intact cells (D). *** p<0.001, ** p<0.01. Scale: 200 µm.
Thus, this work shows that gal-9 mediates the adhesion of Jurkat cells to EA.hy 926 by binding to their H-glycan. Taking into account that the level of expression of galectins in the glycocalyx of lymphocytes is precisely regulated, the mechanism found in the model system is apparently also involved in the adhesion of blood lymphocytes to the endothelium.
The work was published in the journal Biomolecules.
september 25, 2023


