Press-room / news / Science news /
NMR spectroscopy reveals patterns and thermodynamic parameters of dimerization of β- hairpin antimicrobial peptides in the membrane
The staff of the Laboratory of structural biology of ion channels and the Science–Educational center for the first time studied the thermodynamics of the dimerization process of a β-hairpin peptide in the membrane-mimicking environment of detergent micelles using the example of the antimicrobial peptide (AMP) capitellacin of the marine polychaete Capitella teleta. The study also describes the mechanism of capitellacin action on bacterial membranes. The results of the work were published in the journal Biomolecules.
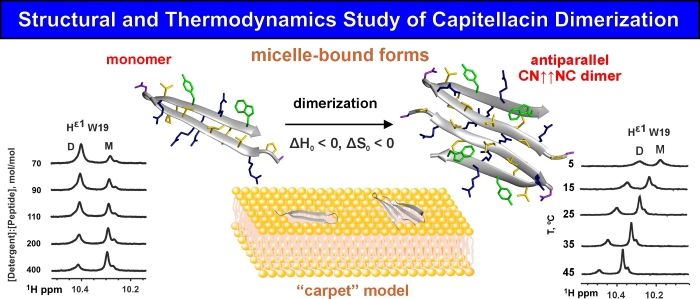
Using NMR spectroscopy, the structures of two forms of capitellacin were determined – a monomeric β-hairpin and a dimer, formed by antiparallel association of the N-terminal β-strands of the peptide. Transitions between these forms occurred with the changes of the temperature and the peptide to detergent molar ratio. The dimerization of β-hairpin peptides in the membrane-like environment occurs due to the interaction of polar or weakly hydrophobic regions and depends more on the intermolecular interactions (hydrogen bonds, electrostatic and van der Waals interactions) than on the hydrophobic effect. Dimerization is characterized by a negative change in enthalpy and entropy.
The topology of both forms of capitellacin in the micelles and the distribution of polar and hydrophobic regions on the molecular surfaces suggested that capitellacin disrupts the membrane integrity according to the “carpet” mechanism. This explains the lower antibacterial, hemolytic and membrane permeabilization activities of capitellacin in comparison with other β-hairpin peptides, which can form pores in the target membranes. The comparison of structural and physicochemical properties of known β-hairpin AMPs in monomeric and dimeric forms revealed positive correlation between the dimer stability, hemolytic activity and hydrophobicity of the AMP surface. Surprisingly weak positive correlation between total charge of the peptide and its hemolytic activity was also observed.
The results obtained allow more accurate description of the folding of β-barrel membrane proteins and the formation of oligomeric transmembrane pores by β-structural peptides. We believe that studying the relationship between the spatial structures and biological activities of AMPs will help to develop new antibiotic compounds required for the treatment of bacterial infections.
april 10