Лаборатория механизмов генной экспрессии
|
Отдел функционирования живых систем Руководитель: Шпаковский Г.В. |
Основные направления работы лаборатории: изучение молекулярной эволюции транскрипционного аппарата и механизмов экспрессии генов у эукариотических организмов (от дрожжей до человека) [J. Mol. Biol., 2000, 295: 1119-1127; Биоорган. химия, 2004, 30: 621-625; Генетика, 2010, 46: 1254-1257; Cell andTissue Biology, 2013, 7: 314-319] и изучение новой, недавно обнаруженной нами прогестероновой системы гормональной регуляции у высших растений [патент РФ № 2237717 от 10.10.2004; Биоорган. химия, 2010, 36: 241-250; Журнал стресс-физиологии и биохимии, 2014, 10: 85-97; BMC Plant Biology, 2017, 17: 189].
В рамках первого направления установлено, что у человека, в отличие от подавляющего большинства эукариот, существуют множественные варианты субъединицы hRPB11 (POLR2J) РНК-полимеразы II и что эти различные изоформы кодируются в геноме Homo sapiens четырьмя разными генами, расположенными на хромосоме 7. Показано, что специфические для человека изоформы hRPB11 содержат в своём составе пептидные участки, характерные для белков системы репарации из семейства PMS2, которые в геноме человека также кодируются множественными генами (см. рис. 1). В ходе дальнейших исследований мы установили, что молекулярная эволюция генных семейств POLR2J и PMS2, кодирующих базовые, незаменимые компоненты двух важнейших (в том числе и для эволюции) молекулярно-биологических систем клетки, транскрипции и репарации ДНК (MMR, mismatch repair), чётко коррелирует (совпадает) с основными этапами дивергенции высших приматов (Lesser & Great Apes) и привела к появлению у человека новых, уникальных белков, таких как минорные субъединицы РНК-полимеразы II hRPB11ba (hRPB11сa) и hRPB11bb (hRPB11сb) [Генетика, 2010, 46: 1254-1257]. С целью прояснения функций этих специфичных для человека изоформ мы установили спектры взаимодействий (интерактомы) этих белков в нервной и лимфоидной тканях Homo sapiens, впервые выявив уникальные белковые партнёры каждой из изоформ (Биохимия, 2011, 76: 1195-1200; Cell and Tissue Biology, 2013, 7: 314-319). Среди обнаруженных партнеров изоформы hRPB11ba (hRPB11сa) оказались субъединицы РНК-полимераза II hRPB3 и hRPB6, транскрипционный фактор ATF4, а также новая изоформа eIF3mβ субъединицы фактора инициации трансляции eIF3m [Биохимия, 2011, 76: 1195-1200]. Выясненные нами белковые взаимодействия eIF3mβуказывают на то, что этот ядерно-цитоплазматический белок вовлечен в высокоэффективную систему синтеза и транспорта мРНК мембранных белков. Возможно, эта система играет важную роль в нейронах, где стандартный хромосомный набор клетки должен обеспечивать синтез достаточного количества белков для гигантских мембранных поверхностей. В качестве первого гена человека, в транскрипции которого участвуют транскрипционные комплексы, содержащие изучаемые нами минорные изоформы субъединицы РНК-полимеразы II hRPB11, выявлен CLN3, кодирующий мембранный белок баттенин. Мутации в этом гене приводят к возникновению болезни Баттена, тяжёлого нейродегенеративного заболевания, в патогенезе которого участвуют специфичные для человека гены семейства POLR2J (Biology Direct, 2018, in press).
Второе направление связано с получением и изучением трансгенных растений с гибридными (Animalia-Plantae) стероидогенными системами. В сотрудничестве с белорусскими учёными (Институт генетики и цитологии НАН Беларуси) мы показали, что уникальный только для животных ключевой фермент стероидогенеза CYP11A1 (Р450scc), катализирующий окислительное расщепление боковой цепи холестерина с образованием общего предшественника всех стероидных гормонов – прегненолона, успешно функционирует в трансгенных растениях табака, наперстянки и томата, повышая их устойчивость к фитопатогенам и абиотическим стрессам и ускоряя процессы роста и развития (Генетика, 2009, 45: 1217-1224; Журнал стресс-физиологии и биохимии, 2014, 10: 85-97). Тем самым впервые продемонстрирована совместимость in vivo даже самых специфичных компонентов систем биосинтеза стероидных гормонов растений и животных. Обусловленное повышенным уровнем эндогенного прогестерона формирование вышеупомянутых фенотипов у полученных трансгенных растений семейств Паслёновые и Норичниковые, экспрессирующих кДНК цитохрома P450scc (CYP11A1) млекопитающих, подразумевает, что прогестерон можно считать очень древним биорегулятором растительных клеток и, пожалуй, первым настоящим гормонов, общим для растений и животных (Биоорган. химия, 2010, 36: 241-250; BMC Plant Biology, 2017, 17: 189). Полученные результаты демонстрируют фундаментальное родство путей биосинтеза стероидных соединений и стероидных регуляторных систем у растений и животных и могут быть использованы в новых биотехнологиях для сельского хозяйства и фармакологии.
В Лаборатории также проводились работы по синтезу и исследованию свойств модифицированных олигонуклеотидов, пригодных в качестве праймеров для кПЦР или стабилизирующих в ДНК и РНК неканонические вторичные структуры – эти соединения могут быть использованы в изучении регуляторных областей генов, а также для детекции и подавления вирусов человека (к.х.н. А.В. Аралов: с апреля 2018 г. – Группа молекулярных инструментов для исследования живых систем).
Лаборатория имеет широкий круг партнеров, как в Институте (Лаборатория оксилипинов, Лабораторией инженерии белка), так и за его пределами: Институт физиологии растений имени К.А. Тимирязева РАН; Московский государственный университет имени М.В. Ломоносова; Всероссийский научно-исследовательский институт сельскохозяйственной биотехнологии, Сколковский институт науки и технологий; Российский государственный аграрный университет – МСХА имени К.А. Тимирязева; Сибирский институт физиологии и биохимии растений СО РАН (г. Иркутск). Совместно с белорусскими партнёрами (Институт генетики и цитологии Национальной Академии наук Беларуси, г. Минск; Белорусский государственный медицинский университет, г. Минск) в рамках совместного проекта РФФИ и БРФФИ изучается механизм интеграции митохондриального цитохрома P450scc (CYP11A1) животных в стероидогенную гормональную систему растений и его влияние на физиологию, размножение и иммунитет (проекты № 16-54-00227 и № 18-54-00038). Кроме того, Лаборатория сотрудничает с Институтом биологии и технологий в Сакле (iBiTec-S: Dr. Ju. Soutourina, профессор M. Werner; Франция) и Страсбургской Высшей Школой Биотехнологии (Dr. M. Vigneron (Франция).
Лаборатория создана в мае 2007 года на базе группы с тем же названием, которая существовала как независимое структурное подразделение ИБХ РАН с декабря 2001 г. В более глубокой исторической ретроспективе лаборатория ведёт свой генезис из подразделений института, которыми руководили в своё время академик Михаил Николаевич Колосов (лаборатория химии продуктов микробного синтеза, лаборатория химии генов) и профессор Юрий Адольфович Берлин (группа интерлейкинов, лаборатория генной экспрессии).
Основным направлением исследований лаборатории является изучение механизмов реализации генетической информации сложных геномов на примере эволюции «молодых», человек-специфичных генов-паралогов POLR2J и hPMS2, кодирующих новые изоформы незаменимых субъединиц аппарата транскрипции и MMR-системы репарации Homo sapiens. В настоящее время основные усилия направлены на установление взаимодействий in vivo и детализацию функций в живой клетке минорных изоформ субъединицы hRPB11 (POLR2J): hRPB11ba, hRPB11bb и hRPB11ca, hRPB11cb, кодируемых соответственно специфичными для человека генами POLR2J2 и POLR2J3.
Важнейшими предпосылками для данной работы было обнаружение того факта, что у человека, в отличие от подавляющего большинства эукариотов, существуют множественные варианты субъединицы hRPB11 (POLR2J) РНК-полимеразы II и что эти различные изоформы кодируются в геноме Homo sapiens четырьмя разными генами, расположенными на хромосоме 7 (Биоорган. химия, 2004, 30: 621-625). Впервые данные нами в этой публикации названия генов POLR2J1–POLR2J4, их основные характеристики и классификация кодируемых ими изоформ в настоящее время признаны HUGO и всем научным сообществом. Очень важной «функциональной» предпосылкой было обнаружение Г.В. Шпаковским того факта, что из трёх основных типов изоформ субъединицы hRPB11 (POLR2J) человека (мажорная hRPB11a и минорные hRPB11ba и hRPB11bb) только МИНОРНАЯ hRPB11ba способна, пусть и с определёнными трудностями, комплементировать нулевую аллель Drpb11-HIS3 Sacharomyces cerevisiae (BMC Mol. Biol., 2001, 2: 14). Проведённый нами филогенетический анализ семейств генов POLR2J (RPB11) и PMS2 приматов ясно показал, что в эволюции обоих этих генных семейств имеются стадии, специфичные для человека. Определена анатомия всех четырёх POLR2J и всех шестнадцати PMS2-подобных генов человека, содержащих от 4 до 16 экзонов каждый, проводится работы по изучению тканеспецифичности экспрессии важнейших из кодируемых ими мРНК в норме и патологии (при некоторых формах рака). С помощью генетических (дрожжевая двухгибридная система, мутагенез, супрессорный анализ) и биохимических (соосаждения белков из клеточных лизатов, иммунопреципитация) подходов изучаются белки, взаимодействующие с человек-специфичными изоформами субъединицы hRPB11 (POLR2J), hRPB11ba (hRPB11ca) и hRPB11bb (hRPB11cb), в нервной (эмбриональный мозг) и иммунной (клеточная линия Jurkat) тканях человека. Спектр выявленных нами на сегодняшний день взаимодействий hRPB11ba и hRPB11сa показывает, что эти белки являются минорными изоформами субъединицы РНК-полимеразы II hRPB11 и входят в состав специфичных
РНК-полимеразных комплексов, по-новому регулирующих экспрессию целого ряда важнейших генов человека – нами впервые обнаружены некоторые из таких генов, в частности CLN3 (Biology Direct, 2018, in press). Изучаются также партнёры и функции in vivo впервые обнаруженным нами (среди партнёров минорных изоформ субъединицы hRPB11 РНК-полимеразы II Homo sapiens) белков протеома человека: eIF3mb (Биохимия, 2011, 76: 1195-1200), COMMD4d и DROSHPA (Цитология, 2013, 55: 172-177). В перспективе эти исследования могут привести к разработке новых подходов к диагностике и мониторингу ряда онкологических и неврологических заболеваний человека.
Для изучения механизма интеграции митохондриального цитохрома P450scc (CYP11A1) животных в стероидогенную систему растений и его влияния на физиологию, размножение и иммунитет трансгенных растений томата и табака, экспрессирующих полноразмерную кДНК CYP11A1 быка, ведётся работа по установлению локализации белка CYP11A1 в клетках трансгенных растений, изучаются его функциональные партнёры в протеомах томата (известен и хорошо аннотирован полный геном), табака (нами создана представительная клонотека кДНК трансгенного по CYP11A1 растения) и наперстянки (единственное растение, для которого описана реакция отщепления боковой цепи холестерина с превращением его в прегненолон и предсказаны основные пути биосинтеза и дальнейших превращений прогестерона), анализируются фенотипы и физиология новых поколений (T3 и T4) трансгенных растений томата. Анализируются также партнёры и функции охарактеризованных нами ранее адренонодоксинподобных ферредоксинов (MFDX1 и MFDX2) табака, томата и наперстянки (Журнал стресс-физиологии и биохимии, 2014, 10: 85-97) – компонентов митохондриальной электронтранспортной цепи растений и возможных партнёров цитохрома P450scc (CYP11A1) в их протеоме. Изучение трансгенных растений, экспрессирующих гены стероидогенных белков животных, представляется актуальной научной и прикладной задачей. С одной стороны, эти растения являются удобной моделью для изучения новых, ещё по-настоящему не охарактеризованных, путей гормональной сигнализации у растений. С другой стороны, подход, основанный на применении генов белков стероидогенеза животных, может привести к созданию трансгенных растений, обладающих улучшенными с точки зрения сельского хозяйства признаками, а в случае лекарственных растений (наперстянка) – свойствами, важными для производства медицинских препаратов из растительного сырья. Не исключено также и то, что культуры тканей этих трансгенных растений могут найти биотехнологическое применение для осуществления трансформации различных стероидных соединений или их наработки.
- Впервые продемонстрирована функциональная взаимозаменяемость in vivo отдельных субъединиц эукариотических РНК-полимераз между эволюционно далёкими организмами и проведено первое систематическое исследование взаимозаменяемости in vivo (в клетках дрожжей Saccharomyces cerevisiae) субъединиц ядерных РНК-полимераз I, II и III эволюционно отдалённых эукариотических видов: Schizosaccharomyces pombe и Homo sapiens. (Gene, 1994, 147: 63-69; Mol. Cell. Biol., 1995, 15: 4702-4710; J. Mol. Biol., 2000, 295: 1119-1127; Nucleic Acids Res., 2006, 34: 3615-3624).
- Впервые показано функциональное родство малых субъединиц РНК-полимераз архей (надцарство Archaea) и эукариот (Eucarya) и впервые методом межвидовой комплементации клонированы кДНК, кодирующие незаменимый компонент многосубъединичных ферментов транскрипции (общую субъединицу Rpb10
РНК-полимераз I-III делящихся дрожжей) и белковый фактор Fet5 (Gpn3) Schizosaccharomyces pombe, ставший прототипом нового семейства GTP/ATP–связывающих белков (Gpn1-3), играющих незаменимую роль в транспорте
РНК-полимеразных комплексов из цитоплазмы в ядро клетки (Биоорган. химия, 1997, 23: 110-117; Биоорган. химия, 1997, 23: 234-237; J. Biol. Chem., 1999, 274: 8421-8427). Эти работы удостоены Главной премии издательства МАИК «Наука» за 1998 г. по группе биологических наук (Вестник РАН, 1999, т. 69, № 11, с. 1054). - Установлен субъединичный состав РНК-полимераз I и III Schizosaccharomyces pombe, клонированы и функционально охарактеризованы кДНК и гены, кодирующие все субъединицы этих ферментов (Current Genet., 1999, 36: 208-214; Биоорган. химия, 1999, 25: 791-796; Молекулярная биология, 2002, 36: 3-26; Nucleic Acids Res., 2006, 34: 3615-3624).
- При активном участии лаборатории завершено секвенирование и аннотирование генома делящихся дрожжей Schizosaccharomyces pombe: в рамках международного проекта, благодаря полученным в нашей лаборатории клонам pYUK71, pYUL23 и pYUG7, в трёх локусах восстановлена непрерывность нуклеотидной последовательности хромосомы I, cовместно с сотрудниками Сенгеровского Центра (Хинкстон, Великобритания) лаборатория была ответственной за аннотирование компонентов аппаратов транскрипции и трансляции Schizosaccharomyces pombe. Впервые российская лаборатория отмечена как полноправный участник проекта полного секвенирования генома эукариотического организма (Nature, 2002, 415: 871-880). Эта работа отмечена в числе главных научных достижений РАН за 2001 г. по разделу «Науки о жизни.
Физико-химическая биология» («Отчёт о деятельности РАН в 2001 году /Важнейшие итоги/», с. 43). - С помощью мутагенеза консервативных аминокислотных остатков определен участок (SF/YGGLLM), наиболее важный для функции in vivo общей субъединицы РНК-полимераз I-III Rpb8. С использованием супрессорного анализа и дрожжевой двухгибридной системы показано взаимодействие Rpb8 с субъединицами Rpa1, Rpb1, Rpc1 и Rpb6. Установлено, что N-концевой домен фактора элонгации TFIIS взаимодействует с компонентом Медиатора Med13 и субъединицей Spt8 комплекса SAGA и важен для стабилизации инициаторного комплекса РНК-полимеразы II на промоторах (в сотрудничестве с учёными из Отдела биохимии и молекулярной генетики Центра Атомной Энергии в Сакле [SBGM], Франция (Prof. P. Thuriaux): Mol. Cell. Biol., 2001, 21: 6056-6065; EMBO J., 2004, 23: 4232-4242).
- Впервые (совместно с Институтом генетики и молекулярной и клеточной биологии — Иллкирш, Франция, Dr. M. Vigneron) установлено, что одна из субъединиц ядерных РНК-полимераз кодируется целым семейством генов
(BMC Mol. Biol., 2001, 2: 14). Показано, что один из начальных этапов сборки
РНК-полимеразы II, образование гетеродимера субъединиц Rpb11 и Rpb3, существенно отличается у дрожжей и человека: С-концевой участок субъединицы Rpb11 абсолютно необходим при сборке дрожжевого фермента, но не является критическим для гетеродимеризации hRPB11 и hRPB3. В то же время для сборки РНК-полимеразы II человека в сравнении со сборкой фермента дрожжей гораздо более существенна целостность a–мотива субъединицы Rpb11, расположенного в середине N-концевой части белка (Nucleic Acids Research, 2005, 33: 3582-3590). Охарактеризованы три изоформы субъединицы hRPB11 (POLR2J) человека и выяснен механизм образования этих изоформ (Биоорган. химия, 2004, 30: 621-625). - Изучена молекулярная эволюция двух специфичных для приматов генных семейств: POLR2J системы транскрипции и PMS2 системы репарации MMR. Установлено, что появление и совершенствование генетической структуры каждого из этих семейств чётко коррелируют с основными этапами биологической эволюции высших приматов. Показано, что гены PMS2 и POLR2J могут рассматриваться в качестве достоверных молекулярных маркеров антропогенеза. Предложена гипотеза о трёхкомпонентной белковой системе, продуцируемой PMS2-подобными генами человека. Сформулированы принципы каскадного усиления на поздних стадиях эволюции приматов, которое могло обеспечить ускоренную молекулярную эволюцию человека (Доклады Академии наук, 2006, 408: 699-703; Генетика, 2010, 46: 1254-1257).
- Определён основной спектр белковых партнёров специфичных для человека изоформ субъединицы РНК-полимеразы II hRPB11 (POLR2J) – hRPB11ba, hRPB11сa и hRPB11bb, hRPB11cb. Установлено, что минорные изоформы hRPB11ba и hRPB11ca взаимодействуют сразу с несколькими субъединицами фактора инициации трансляции hEIF3: eIF3a, eIF3i, eIF3ma и eIF3mb, что указывает на существование у Homo sapiens нового типа координации транскрипции с последующими этапами генной экспрессии (процессинг и транспорт мРНК из ядра в цитоплазму к транслирующим полисомам) (Биохимия, 2011, 76: 1195-1200). Среди партнёров изоформ hRPB11bb и hRPB11cb помимо субъединицы РНК-полимеразы II hRPB6 (POLR2F) и кóрового компонента белкового комплекса экзонных сочленений Y14 (RBM8A) обнаружен ряд белков, участвующих в биогенезе микроРНК, в том числе новый, ранее не описанный вариант инициирующей нуклеазы процессинга микроРНК DROSHA, что указывает на существование особых путей сопряжения процессов транскрипции и
РНК-интерференции в ядрах клеток человека (Цитология, 2013, 55: 172-177). - Впервые продемонстрировано функциональное родство стероидогенных систем животных и растений in vivo: экспрессия кДНК гена CYP11A1, кодирующего ключевой фермент стероидогенеза животных цитохром P450scc повышает иммунитет (устойчивость к фитопатогенам) и ускоряет процессы роста и развития трансгенных растений табака, наперстянки и томата. Показано наличие у этих растений двух андренодоксиноподобных [2Fe-2S]-ферредоксинов митохондриального типа (патент РФ № 2237717 от 10.10.2004; Генетика, 2009, 45: 1217-1224, Биоорган. химия, 2010, 36: 241-250; Журнал стресс-физиологии и биохимии, 2014, 10: 85-97; BMC Plant Biology, 2017, 17: 189). Результаты этих работ вошли в перечень важнейших научных достижений РАН 2009 г. (Отчётный доклад Президиума РАН «Научные достижения РАН в 2009 году», с. 220).
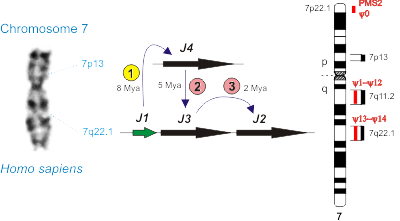
Mapping on the human chromosome 7 several genes that are important for primate evolution: four POLR2J paralogues (J1–J4) and sixteen PMS2 paralogues (PMS2, ψ0, ψ1–ψ14). Different stages (1, 2 and 3) of POLR2J amplification are shown; two of them are specific for Homo sapiens (marked with pink circles). Mya — million years ago.
| ФИО | Должность | Контакты |
|---|---|---|
Ранее здесь работали | ||
| Клыков В.Н., к.х.н. | ||
| Прошкин С.А. | ||
| Словохотов И.Ю. | ||
| Шаршавина Е.А. | ||
| Шпаковский Д.Г. | ||
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Шпаковский Г.В.
Москва, ул. Миклухо-Маклая, 16/10 — На карте
 Загрузка...
Загрузка...
