Группа нанобиоинженерии
|
Руководитель: Некрасова Оксана Васильевна |
Работа группы направлена на биоинженерное конструирование рекомбинантных белков и полипептидов, разработку методов их выделения и ренатурации, изучения их свойств с привлечением современных физико-химических методов и методов нанотехнологии.
Группа сотрудничает с Лабораторией оптической микроскопии и спектроскопии биомолекул, Группой молекулярных инструментов для нейробиологии, Лабораторией молекулярной токсинологии, а также с подразделениями других учреждений – кафедрой биоинженерии биологического факультета МГУ имени М.В. Ломоносова, лабораторией физико-химических основ рецепции ИБХФ им. Н.М. Эмануэля РАН.
Группа нанобиоинженерии образована в 2010 году в составе Отдела биоинженерии ИБХ РАН.
- Биоинженерия клеточной мембраны целых клеток – биоинженерное конструирование и встраивание в бактериальную мембрану рекомбинантных мембранных рецепторов, изучение их лиганд-связывающих свойств в составе клеточной мембраны с использованием флуоресцентных методов детекции.
- Разработка биоинженерных подходов к получению рекомбинантных мембранных светочувствительных белков с целью исследования их структурной организации и особенностей фотохимических превращений.
- Разработка биоинженерных методов получения растворимых белковых и пептидных лигандов в функционально-активной форме с целью изучения их взаимодействия с рецепторными белками.
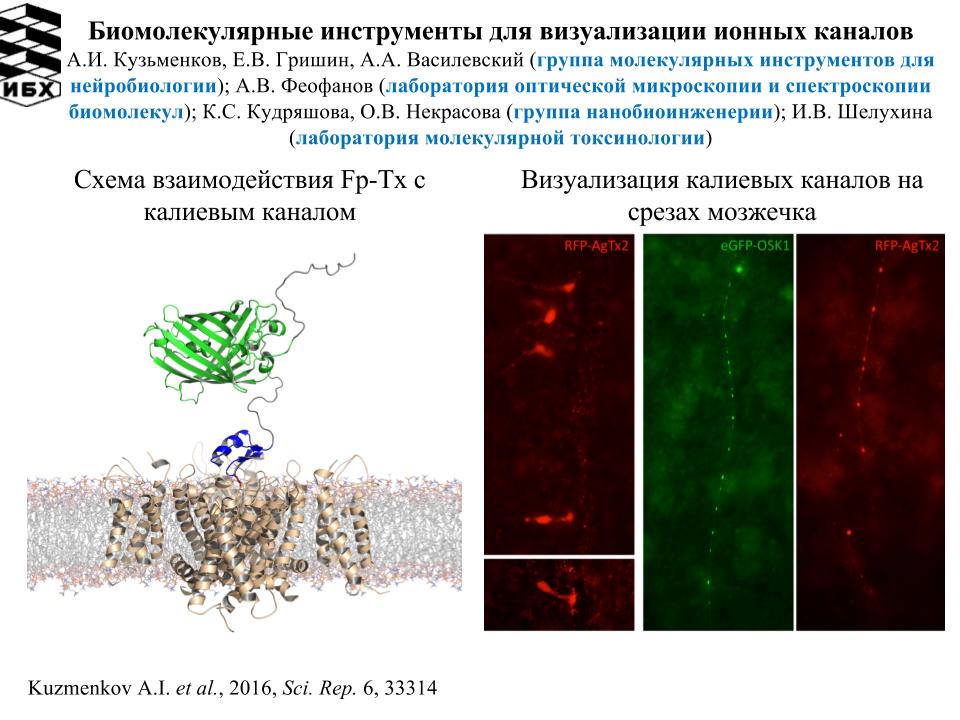
1. Разработана биоинженерная тест-система изучения взаимодействия калиевых каналов с лигандами. Тест-система основана на использовании флуоресцентно-меченых пептидных зондов и гибридных калиевых каналов, встроенных в бактериальную мембрану целых клеток. Детекция лиганд-рецепторных взаимодействий осуществляется методом лазерной сканирующей конфокальной микроскопии (ЛСКМ). С помощью тест-системы в ядах животного происхождения найдены и охарактеризованы новые пептидные блокаторы калиевых каналов Kv1.1, Kv1.3, имеющих важное биомедицинское значение. С привлечением методов молекулярного моделирования проводится изучение молекулярных основ взаимодействия пептидных токсинов с калиевыми каналами, осуществляется конструирование мутантных форм пептидных токсинов с повышенной избирательностью действия в отношении канала-мишени. Разработан принцип создания генокодируемых флуорецентных лигандов калиевых каналов с целью их использования в качестве флуоресцентных зондов при изучении связывания пептидных блокаторов с каналами, а также для визуализации калиевых каналов в клетках и тканях. Работа проводится совместно с подразделениями ИБХ РАН – лабораторией оптической микроскопии и спектроскопии биомолекул, группой молекулярных инструментов для нейробиологии, лабораторией молекулярной токсинологии, а также с кафедрой биоинженерии биологического факультета МГУ имени М.В. Ломоносова.

2. Разработан биоинженерный метод супер-продукции рекомбинантного бактериородопсина (из Halobacterium salinarum) в системе экспрессии E.coli. Фотохимические свойства рекомбинантного бактериородопсина аналогичны свойствам мономерной формы природного бактериородопсина. Рекомбинантный бактериородопсин и его мутантные формы используются для изучения первичных стадий фотоцикла и процессов переноса энергии методом фемтосекундной абсорбционной спектроскопии (совместно с лабораторией физико-химических основ рецепции ИБХФ им. Н.М. Эмануэля РАН).
3. Разработаны новые эффективные биоинженерные методы получения функционально-активных рекомбинантных лигандов: дисульфид-богатых пептидных токсинов из яда скорпионов; эфрина А1 - лиганда эфриновых рецепторов. Полученные рекомбинантные пептиды и белки используются в различных исследованиях лиганд-рецепторных взаимодействий.
| ФИО | Должность | Контакты |
|---|---|---|
Ранее здесь работали | ||
| Некрасова Оксана Васильевна, к.б.н. | ||
| Тихонов Р.В., к.х.н. | ||
| Крюкова Елена Александровна | ||
| Кудряшова К.С., к.б.н. | ||
| Якимов С.А. | ||
| Бирих К.Р., к.б.н. | ||
 Загрузка...
Загрузка...Научные проекты
 Загрузка...
Загрузка...Некрасова Оксана Васильевна
Москва, ул. Миклухо-Маклая, 16/10 — На карте
 Загрузка...
Загрузка...
