Laboratory of optical microscopy and spectroscopy of biomolecules
The Laboratory studies biologically active molecules, membranous and membrane-active proteins and develops new methods of optical microscopy and spectroscopy to support these studies. Developed methods are used to study the action mechanisms and structural-functional interrelationships of investigated molecules at different levels of structural organization: molecular, cellular and tissue.
The methods of microscopy of single-molecules and their complexes are developed in the Laboratory using the unique scientific equipment. It includes an installation for one-photon and multiphoton 4Pi superresolution microscopy and an installation for fluorescence microscopy based on the effect of total internal reflection. Both installations have got a high sensitivity, allowing registering fluorescent signals even from single molecules.
Experimental installation for confocal laser microspectroscopy was created in the Laboratory. Also, the method of confocal microspectroscopy and reconstruction of spectral images (COMIRSI) was developed that provided possibility to identify and study molecular interactions of biologically active compounds in living cells with a three-dimensional submicron spatial resolution. The experimental installation and the COMIRSI method are widely used in the development, scientific and clinical studies of new Russian photosensitizers for photodynamic cancer therapy, as well as in the studies of membrane and membrane-active proteins.
The Laboratory is open for cooperation with the departments of the Institute and other scientific organizations.
The development of analytical systems based on hybrid ion channels for the search and study of potassium channel blockers is conducted jointly with the Group of nanobioengineering. A search and study of potassium channel blockers in scorpion venoms are carried out in collaboration with the Molecular Instruments for Neurobiology Group. The functional properties of toxins from snake venom are studied together with the Laboratory of Molecular Toxinology.
Also, the Laboratory conducts joint studies with a number of other scientific organizations. Study of photosensitizers for photodynamic anti-cancer and antimicrobial therapy and optimization of their properties is carried out in cooperation with the Moscow Technological University, the P.A. Herzen Moscow Oncology Research Institute and the State Scientific Center "NIOPIK". Studies of structural rearrangements in nucleosomes under the influence of various nuclear proteins using the methods of fluorescence microscopy of single molecules and their complexes are carried out together with the Bioengineering Department, the Laboratory for the Regulation of Transcription and Replication of the Biological Faculty of Moscow State University and colleagues from Fox Chase Cancer Center (USA). The structure optimization of conjugates of boron-containing nanoparticles and natural porphyrins is conducted jointly with the Moscow Technological University and INEOS RAS.
The laboratory collaborates with foreign scientific organizations. Biomedical nanosensors for the diagnosis and treatment of breast cancer are being developed together with the University of Tours (France) and the Rhine-Waal University of Applied Sciences (Germany).
The Laboratory employees teach the basics of optical microscopy to students of the ESC of the IBCh RAS and the Department of Bioengineering of the Biological Faculty of the Moscow State University. Each year, several bachelor's and master's works as well as scientific research of graduate students and young scientists are conducted. under the supervision of the Laboratory employees
The Laboratory of optical microscopy and spectroscopy of biomolecules was established in 2005. It is a part of the Department of Structural Biology, which arose as a result of reorganization of the Laboratory of Instrumental Methods of Analysis. This reorganization was conducted due to the initiative of professor A.S. Arsenyev, who is the current head of the Department.
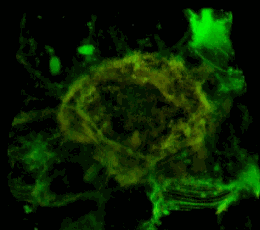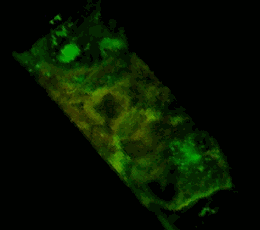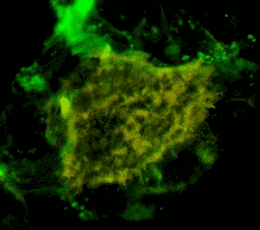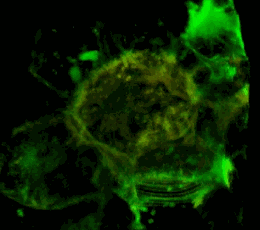
A three-dimensional confocal image of HEK293 cells in which a stable expression of the EphA2 receptor of EphA2 fused to a cyan fluorescent protein (shown in green) is achieved, together with EphA2 fused to a yellow fluorescent protein (shown in red). The coincidence of the two structures localization is shown in yellow. The cell model is used to study the mechanisms of activation and dimerization of Efrin receptors using the method of resonant fluorescence energy transfer (FRET).
- Cellular bioengineering systems and methods of their applications using fluorescence microscopy have been developed. They are used to study pore blockers of voltage-gated potassium channels and to search for peptide blockers in natural venoms.
- Using fluorescent proteins and scorpion toxins, the high-affinity conjugates were designed that interact selectively with Kv1 channels and can be used for their visualization.
- New high-affinity channel blockers of Kv1.x (x = 1-3.6) were discovered in scorpion venoms and characterized by activity.
- Recombinant peptide-blockers of Kv-channels having improved selectivity were created. Molecular models of peptide-blockers complexes with Kv-channels were constructed.; Interaction interfaces and amino acid residues affecting the force and selectivity of interactions were described.
- Methods for studying single molecules and their complexes in the free diffusion regime and in the immobilized state have been developed based on Forster resonance energy transfer microscopy. Applicability of these methods for investigation of structural changes of nucleosomal DNA in complexes with different protein factors was demonstrated.
- Structures of new conjugates of boron-containing nanoparticles and natural porphyrins have been optimized that provided delivery of more than 1 billion boron atoms per cancer cell. In this way, applicability of new nanoconjugates for boron-neutron capture therapy (BNCT) was provided. Nanoconjugates are active photosensitizers and cause photoinduced death of tumor cells at nanomolar concentrations. New conjugates are perspective multifunctional agents for photodynamic therapy, BNСT and fluorescent cancer diagnostics.
- It has been established that the key structural element determining high accumulation of chlorin e6 conjugates with cobalt bis(dicarbolide) nanoparticle in cancer cells is the amino-polyalkyl-amine linker that connects a porphyrin chromophore to nanoparticle. Photocytotoxicity of conjugates is based on photoinduced lipid peroxidation, lysosomes permeabilization, and proteases activation in cytoplasm of cells.
| Fullname | Position | Contacts |
|---|---|---|
| Alexey Feofanov, AP, D.Sc | pr. r. f. | |
| George Sharonov, Ph.D. | s. r. f. | |
| Anastasia Efremenko | r. f. | |
| Anastasia Ignatova | r. f. | |
| Dzhumaniyazova I.H. | j. r. f. | |
| Orlov N.A. | j. r. f. | |
| Shmatin I.I. | res. eng. | |
| Korabeinikova V.N. | eng. | |
| Likov B.Y. | eng. | |
Previously worked here | ||
| Astapova M.V., Ph.D. | ||
| Denisova K.R. | ||
| Feofanova A.E. | ||
| Ovcharenko M.D. | ||
| Sokolova M.A. | ||
 Loading...
Loading...Scientific projects
 Loading...
Loading...Alexey Feofanov
Russia, Moscow, Ul. Miklukho-Maklaya 16/10 — On the map
Scopus: 7003518369, ResearcherID: K-3082-2012
 Loading...
Loading...
