Press-room / news / Science news /
The way to study of tyrosine kinases receptor
Receptor tyrosine kinases - one of the key links in a cell signaling system. They play a key role in the regulation of intercellular interactions, cell proliferation, morphogenesis and metabolism. Their dysfunctions are associated with such socially significant diseases as cancer and diabetes. In M.M. Shemyakin and Yu.A. Ovchinnikov Institute of bioorganic chemistry several research groups perform researches, concerning receptor tyrosine kinases.
In the Laboratory of Biomolecular NMR Spectroscopy (the head of the Laboratory is Prof. Alexander Arseniev) together with the Department of Bioengineering IBCh RAS a number of experiments with receptor tyrosine kinase VEGFR-2 were performed. This tyrosine kinase belongs to the family of vascular endothelial growth factor receptors, regulates the process of angiogenesis and is one of the key targets for anticancer drugs.
The distinguishing feature of receptor tyrosine kinases is a transmembrane segment, connecting an extracellular part and intracellular domains. In the experiment scientists have studied the role of the transmembrane segment receptor in the processes of receptor activation and found a series of mutations that are able to induce ligand-independent activation of VEGFR-2. They found VEGFR-2 with a "cut" extracellular domain as well. To carry out a detailed study of the transmembrane domain the scientists have developed a special technique that enables to measure the free energy of transmembrane spirals association in micellar media by NMR spectroscopy with high-resolution, that allowed to measure the value of the free energy of dimerization and, where it was possible, trimerization, for all three objects. Based on the data of mutagenesis, NMR spectroscopy and obtained spatial structures, the mechanism of VEGFR-2 activation, implying the existence of an inactive state of the receptor dimer.
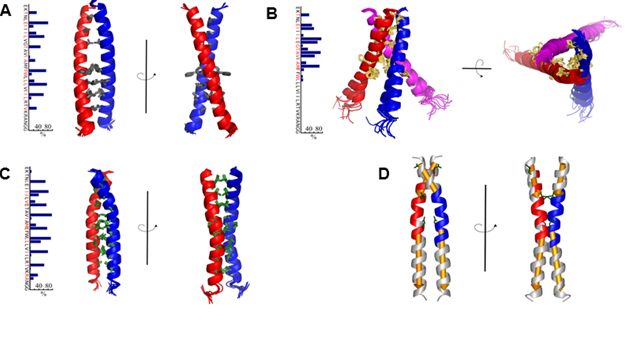
Fig. 1. The spatial structure of the TM domain dimer VEGFR-2 wild type (A), trimer TMU VEGFR V769E (B), and TMP dimer VEGFR-2 G770E / F778E (C). D, The structure of the dimer TM domain VEGFR-2 G770E / F778E, painted in accordance with the intramolecular mobility.
To understand the functioning of the receptor tyrosine kinase is necessary to study the mechanism of signal transduction activation from the extracellular side of the membrane into the cytoplasm. It is known that extracellular interaction of EphA2 ligand receptor activator causes receptor dimerization, which occurs with both the extracellular and cytoplasmic domains. Dimerization promotes EphA2 receptor phosphorylation, which is a key step in the launch of intracellular signaling cascades. The role of transmembrane domain receptors was unknown. Development of new techniques of flow cytometry by PhD. Georgiy Sharonov, and their application to the study of EphA2 receptor mutations inserted in the transmembrane domain, allowed to find the right answer. As a result of research carried out at the Laboratory of optical microscopy and spectroscopy of biomolecules on leadership PhD. Alexey Feofanov, it was found that the transmembrane domains of EphA2 receptor form two different types of dimers structure which stabilize the active or an inactive receptor conformation. The high density of receptors in the membrane promotes activation of EphA2 even in the absence of ligand. Dimer conformation of the transmembrane domain modulates ligand-dependent and ligand-independent mechanisms of EphA2 activation. First time experimental data showed that switching between the two types of dimers transmembrane domain is an active element of the mechanism of signal transmission in EphA2 receptors.
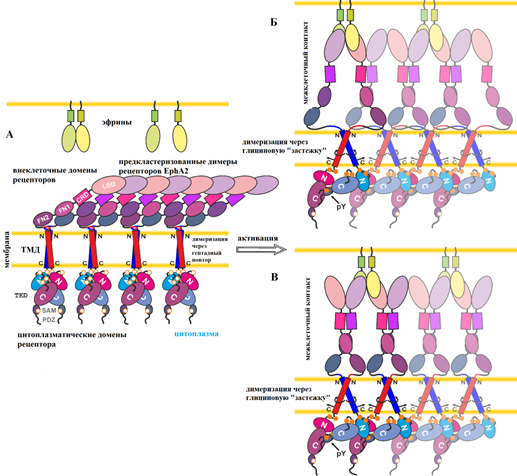
Fig. 2. Mehanism EphA2 activation with the new data on the two alternative configurations TMP dimers. Figure adapted from an article (Sharonov et al. JBC 2014).
Laboratory of Cell Biology receptors IBCh RAS led by PhD Alexander Petrenko proposed model of receptor tyrosine kinases activation that has been described in the article in the international scientific journal Biochimie. The new model of of receptor tyrosine kinases activation is based on the analysis of the activity of the mutant receptor. A senior fellow at the Laboratory of Cell Biology PhD Igor Deyev developed and first time used in a series of experiments the new method of receptor activation - autophosphorylation in vitro, which allows to compare the activation of the receptor and its mutants quantitatively. Using this technique, researchers have shown that the receptor has two main sites involved in its response to changes in pH. The first site is located between domains L1C of one monomeric subunit and FnIII-2 domain, and FnIII-3 subunit from a second monomer, and the second site is between L2 and FnIII-1 domains. The first site has a greater significance for the successful activation of the receptor. Further study of tyrosine kinases receptors can make a significant contribution to the understanding of the processes, occurring both in physiological activation of the insulin receptor and in pathological disorders, occurring in patients with diabetes.
april 23, 2015

