Press-room / news / Science news /
PRMT5 methylome profiling uncovers a direct link to splicing regulation in acute myeloid leukemia
Protein arginine methyltransferase 5 (PRMT5) belongs to the class II arginine methyltransferases and catalyzes monomethylation and symmetrical dimethylation of arginines on proteins. It has recently emerged as a promising cancer drug target, and two PRMT5 inhibitors are currently in clinical trials for a range malignancies. Despite the recognized therapeutic potential, it is unclear which PRMT5 functions underlie its oncogenic activity.
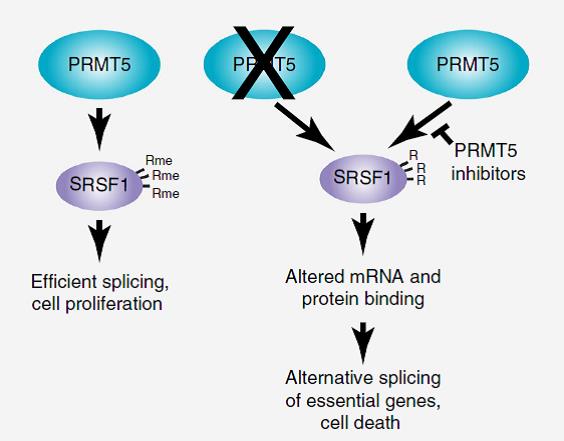
Scientists from the University of Copenhagen, University of Southern Denmark, Memorial Sloan Kettering Cancer Center in New York, USA, Belarusian State University together with the scientist from Laboratory of Bioinformatic methods in Chemistry and Biology, IBCH RAS Kovalchuk S.I. studied the role of PRMT5 protein in cell functioning and its mode of action in leukemia in the model of human acute myeloid leukemia (AML) cells. PRMT5 activity was connected to SRSF1 protein direct regulation through Arg methylation. SRSF1 is an alternative splicing regulator and PRMT5 inhibition changes splicing patterns for a range of essential genes, which leads to AML cell death. The results of the work can provide potential biomarkers for the treatment response to PRMT5 inhibitors. The work was published in Nature Structural and Molecular Biology.
october 29, 2019

