Press-room / news / Science news /
New structural data made it possible to decipher the mechanism of human oncochannel TRPV6 inhibition by the natural phytoestrogen genistein
Calcium-selective oncochannel TRPV6 is the major driver of cell proliferation in human body and its overexpression was found in some of the most severe human cancers, including leukemia, breast, prostate, colon, ovarian, thyroid, and endometrial cancers. While significant effort has been invested in the development of synthetic TRPV6 inhibitors, natural channel blockers have been largely neglected despite of their pharmacological value.
Researchers from the Laboratory of Biomolecular Modeling together with colleagues from Columbia University (New York, USA) have identified structural aspects of the interaction of TRPV6 with genistein, that is a natural phytoestrogen common in various types of legumes (including soybeans). Earlier in clinical studies it was shown that the dietary genistein has a range of potential health-beneficial effects, including the ability to inhibit cell invasion and metastasis in various forms of human cancer.
Using a state-of-the art biophysical approach combining cryo-EM, electrophysiology, calcium imaging, protein engineering, and molecular modeling methods, the team of scientists managed to show that genistein, being bound to TRPV6, works as a pore blocker that changes the structure of the activation gate of the ion channel. The genistein binds with the pore region in two sites (see Fig. 1): in site 1 – due to the formation of hydrogen bonds with the residues L571 of diagonally located protein subunits, and in the primary position of site 2, where it interacts with several polar protein groups. Because of these interactions, the pore forming α- helices change their conformations thus breaking of the pore symmetry. In addition, this modifies the structure of the activation gate of the channel. As a result, TRPV6 goes into a non-conductive state. The genistein in the primary position of site 2 is stabilized by another genistein molecule bound in the secondary position, where it is held due to π-stacking interactions with residues W583. The entire molecular complex of the closed TRPV6 pore is additionally stabilized by Zn2+ ions.
The described mechanism of genistein blockade of TRPV6 is unique amongst all previously described mechanisms of TRP channel inhibition by various natural and synthetic antagonists. Considering the potential to using various well-established chemical synthesis pathways of genistein and its derivatives, the obtained results open new avenues for the development of innovative drugs targeting the TRPV6 channel, including anticancer drugs.
The results are published in Nature Communications.
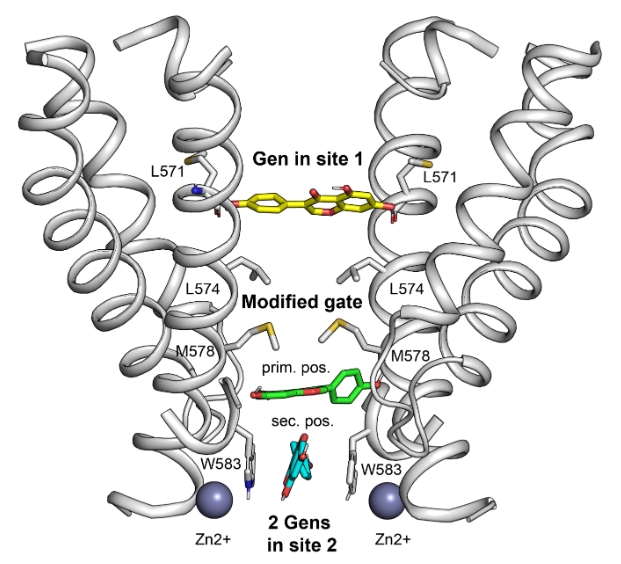
Fig.1. Side-view of the pore of TRPV6 channel blocked by genistein (Gen). The pore forming transmembrane α-helices of the protein are given in cartoon representation (two subunits out of four are shown). The genistein molecule in site 1 is indicated with yellow sticks; while in the primary and secondary position of site 2 – with green and cyan sticks, respectively. The spheres are Zn 2+ ions. The modified activation gates of the channel are placed between sites 1 and 2.
may 23, 2023