Press-room / news / Science news /
The structure of the p75 protein will help fight neurological disorders
Researchers of the Institute of Bioorganic Chemistry (IBCh) of the Russian Academy of Sciences, in joint collaboration with colleagues from the Institute of Health Carlos III (Madrid, Spain) have identified the spatial structure and signal transduction mechanisms of the p75 protein. This study will help understand how to deal with neurological disorders, including Alzheimer’s and Parkinson's. The results of the work are published in the Biophysical Journal and Journal of Biological Chemistry.
Protein p75 is responsible for a wide range of processes in human cerebral neurons: from control of the development and division of nerve cells up to their apoptosis (controlled death). It was known that p75 is composed of several parts (domains), which recognize a cell surface signal and transmit it to the inside. However, the exact way in which the domains interacted and worked together was not known until just recently. The common assumption was that they operate on the snailtong principle: a divergence of the extracellular parts causes a convergence of the intracellular domains. However, researchers of the Laboratory of biomolecular NMR-spectroscopy of IBCh in collaboration with Professor Vilar from the Institute of Health Carlos III in Madrid have ascertained the fact that this is not the case.
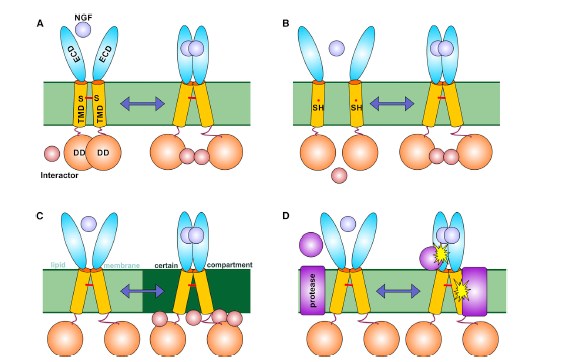
Possible mechanisms of p75NTR activation.
– The p75 neurotrophin receptor, as well as many other membrane proteins, consists of three domains: extracellular, intracellular and membranous, which permeates the cell wall. In its active state, the protein model that we established allowed us to discover that p75 has a completely different structure. The movements of the intracellular and membranous domains are independent from each other, and therefore, the snailtong concept is incorrect, – remarks Konstantin Mineev, PhD, Senior Researcher in Laboratory of biomolecular NMR-spectroscopy of IBCh, co-author of the article.
In addition, as part of the study, the laboratory staff examined the structure of individual protein parts using nanodiscs. Nanodiscs are parts of lipid cell walls surrounded by apolipoproteins (a protein that is active in the transfer of cholesterol and other fats). The scientists placed two protein domains into this more-genuine membrane and determined their 3D-structure by nuclear magnetic resonance spectroscopy. As a result, the system allowed to take into account the effect of the membranes on the work performed by the protein as a whole. The system also demonstrated the signal transduction mechanisms inside nerve cells.
– The study of membrane proteins in structural biology is a difficult task in itself. Primarily, this is due to the complexities involved in the production of proteins and correctly choosing the medium that acts as the cell membrane. And when it comes to the junctions of the transmembrane and fully-functional intracellular domains, embedded to the nanodiscs of a sufficiently large diameter , it becomes a task that only very few can cope with, – comments Alexander Arseniev, professor, Doctor of science, Head of the Structural biology of IBCh.
Until now, researchers could examine only specific domains of this type of proteins. The work done by Alexander Arseniev’s team clarified the exact mechanisms for p75 receptor signaling under conditions of the normal functioning of the nervous system, as well as during pathology. In the future, this will allow the creation and testing of a new generation of effective treatment medication to combat a wide range of neurological disorders such as Alzheimer's and Parkinson's.
may 20, 2016

